Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for UGT1A1 and Atazanavir Prescribing - PubMed (original) (raw)
Practice Guideline
doi: 10.1002/cpt.269. Epub 2015 Nov 9.
M H Court 2, C E Haidar 1, O F Iwuchukwu 3 4, A H Gaur 5, M Alvarellos 6, C Guillemette 7, J L Lennox 8, M Whirl-Carrillo 6, S S Brummel 9, M J Ratain 10, T E Klein 6, B R Schackman 11, K E Caudle 1, D W Haas 12; Clinical Pharmacogenetics Implementation Consortium
Affiliations
- PMID: 26417955
- PMCID: PMC4785051
- DOI: 10.1002/cpt.269
Practice Guideline
Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for UGT1A1 and Atazanavir Prescribing
R S Gammal et al. Clin Pharmacol Ther. 2016 Apr.
Abstract
The antiretroviral protease inhibitor atazanavir inhibits hepatic uridine diphosphate glucuronosyltransferase (UGT) 1A1, thereby preventing the glucuronidation and elimination of bilirubin. Resultant indirect hyperbilirubinemia with jaundice can cause premature discontinuation of atazanavir. Risk for bilirubin-related discontinuation is highest among individuals who carry two UGT1A1 decreased function alleles (UGT1A1*28 or *37). We summarize published literature that supports this association and provide recommendations for atazanavir prescribing when UGT1A1 genotype is known (updates at www.pharmgkb.org).
© 2015 American Society for Clinical Pharmacology and Therapeutics.
Figures
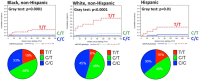
Figure 1
Cumulative incidence of time to bilirubin‐associated discontinuation of atazanavir stratified by UGT1A1 genotype in AIDS Clinical Trials Group protocol A5257 (adapted from ref. 32). Top panels: Lines estimate the cumulative incidence of time to bilirubin‐associated discontinuation of atazanavir, stratified by UGT1A1 rs887829 genotype and self‐reported race/ethnicity. P values are given by Gray's test for testing equality of cumulative incidence functions. Dashed red lines represent rs887829 T/T, dotted green lines rs887829 C/T, and solid blue lines rs887829 C/C. Bottom panels: Proportions of individuals in this analysis with rs887829 T/T, C/T, and C/T genotypes.
Similar articles
- Effect of the UGT1A1*28 allele on unconjugated hyperbilirubinemia in HIV-positive patients receiving Atazanavir: a systematic review.
Culley CL, Kiang TK, Gilchrist SE, Ensom MH. Culley CL, et al. Ann Pharmacother. 2013 Apr;47(4):561-72. doi: 10.1345/aph.1R550. Epub 2013 Apr 2. Ann Pharmacother. 2013. PMID: 23548653 Review. - Association between the UGT1A1*28 allele and hyperbilirubinemia in HIV-positive patients receiving atazanavir: a meta-analysis.
Du P, Wang A, Ma Y, Li X. Du P, et al. Biosci Rep. 2019 May 2;39(5):BSR20182105. doi: 10.1042/BSR20182105. Print 2019 May 31. Biosci Rep. 2019. PMID: 30962262 Free PMC article. - Race/ethnicity difference in the pharmacogenetics of bilirubin-related atazanavir discontinuation.
Leger P, Chirwa S, Nwogu JN, Turner M, Richardson DM, Baker P, Leonard M, Erdem H, Olson L, Haas DW. Leger P, et al. Pharmacogenet Genomics. 2018 Jan;28(1):1-6. doi: 10.1097/FPC.0000000000000316. Pharmacogenet Genomics. 2018. PMID: 29117017 Free PMC article. - Short communication: UGT1A1*28 variant allele is a predictor of severe hyperbilirubinemia in HIV-infected patients on HAART in southern Brazil.
Turatti L, Sprinz E, Lazzaretti RK, Kuhmmer R, Agnes G, Silveira JM, Basso RP, Pinheiro CA, Silveira MF, de Almeida S, Ribeiro JP, Mattevi VS. Turatti L, et al. AIDS Res Hum Retroviruses. 2012 Sep;28(9):1015-8. doi: 10.1089/AID.2011.0261. Epub 2012 May 3. AIDS Res Hum Retroviruses. 2012. PMID: 22050734 - Uridine diphosphate glucuronosyltransferase 1A1.
Steventon G. Steventon G. Xenobiotica. 2020 Jan;50(1):64-76. doi: 10.1080/00498254.2019.1617910. Epub 2019 Jun 3. Xenobiotica. 2020. PMID: 31092094 Review.
Cited by
- Integration of Germline Pharmacogenetics Into a Tumor Sequencing Program.
Hertz DL, Glatz A, Pasternak AL, Lonigro RJ, Vats P, Wu YM, Anderson B, Rabban E, Mora E, Frank K, Robinson DR, Mody RJ, Chinnaiyan A. Hertz DL, et al. JCO Precis Oncol. 2018;2:PO.18.00011. doi: 10.1200/po.18.00011. Epub 2018 Jul 23. JCO Precis Oncol. 2018. PMID: 32832831 Free PMC article. - Incidence of Exposure of Patients in the United States to Multiple Drugs for Which Pharmacogenomic Guidelines Are Available.
Samwald M, Xu H, Blagec K, Empey PE, Malone DC, Ahmed SM, Ryan P, Hofer S, Boyce RD. Samwald M, et al. PLoS One. 2016 Oct 20;11(10):e0164972. doi: 10.1371/journal.pone.0164972. eCollection 2016. PLoS One. 2016. PMID: 27764192 Free PMC article. - CYP2C8*3 and *4 define CYP2C8 phenotype: An approach with the substrate cinitapride.
Campodónico DM, Zubiaur P, Soria-Chacartegui P, Casajús A, Villapalos-García G, Navares-Gómez M, Gómez-Fernández A, Parra-Garcés R, Mejía-Abril G, Román M, Martín-Vílchez S, Ochoa D, Abad-Santos F. Campodónico DM, et al. Clin Transl Sci. 2022 Nov;15(11):2613-2624. doi: 10.1111/cts.13386. Epub 2022 Sep 6. Clin Transl Sci. 2022. PMID: 36065758 Free PMC article. Clinical Trial. - Patterns of pharmacogenetic variation in nine biogeographic groups.
Hernandez S, Hindorff LA, Morales J, Ramos EM, Manolio TA. Hernandez S, et al. Clin Transl Sci. 2024 Sep;17(9):e70017. doi: 10.1111/cts.70017. Clin Transl Sci. 2024. PMID: 39206687 Free PMC article. - Advantages of Array-Based Technologies for Pre-Emptive Pharmacogenomics Testing.
Shahandeh A, Johnstone DM, Atkins JR, Sontag JM, Heidari M, Daneshi N, Freeman-Acquah E, Milward EA. Shahandeh A, et al. Microarrays (Basel). 2016 May 28;5(2):12. doi: 10.3390/microarrays5020012. Microarrays (Basel). 2016. PMID: 27600079 Free PMC article. Review.
References
- Court, M.H. et al Quantitative distribution of mRNAs encoding the 19 human UDP‐glucuronosyltransferase enzymes in 26 adult and 3 fetal tissues. Xenobiotica 42, 266–277 (2012). - PubMed
- Bosma, P.J. et al Bilirubin UDP‐glucuronosyltransferase 1 is the only relevant bilirubin glucuronidating isoform in man. J. Biol. Chem. 269, 17960–17964 (1994). - PubMed
- Strassburg, C.P. Pharmacogenetics of Gilbert's syndrome. Pharmacogenomics 9, 703–715 (2008). - PubMed
- Kadakol, A. et al Genetic lesions of bilirubin uridine‐diphosphoglucuronate glucuronosyltransferase (UGT1A1) causing Crigler‐Najjar and Gilbert syndromes: correlation of genotype to phenotype. Hum. Mutat. 16, 297–306 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL0105198/HL/NHLBI NIH HHS/United States
- U01 HL105198/HL/NHLBI NIH HHS/United States
- P30 AI050409/AI/NIAID NIH HHS/United States
- R24 GM061374/GM/NIGMS NIH HHS/United States
- UL1 TR000445/TR/NCATS NIH HHS/United States
- R24 GM115264/GM/NIGMS NIH HHS/United States
- R01 AI077505/AI/NIAID NIH HHS/United States
- U01 GM061393/GM/NIGMS NIH HHS/United States
- UM1 AI069439/AI/NIAID NIH HHS/United States
- UO1 GM92666/GM/NIGMS NIH HHS/United States
- UM1 AI068616/AI/NIAID NIH HHS/United States
- P30 AI110527/AI/NIAID NIH HHS/United States
- TR000445/TR/NCATS NIH HHS/United States
- R24 GM61374/GM/NIGMS NIH HHS/United States
- U01 GM092666/GM/NIGMS NIH HHS/United States
- U01 AI046339/AI/NIAID NIH HHS/United States
- UM1 AI069418/AI/NIAID NIH HHS/United States
- R01 GM102130/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical