Mutations in the Plasmodium falciparum chloroquine resistance transporter, PfCRT, enlarge the parasite's food vacuole and alter drug sensitivities - PubMed (original) (raw)
Henry M Staines 1, Andrew H Lee 2, Sarah H Shafik 3, Guillaume Bouyer 1 4 5, Catherine M Moore 1, Daniel A Daley 6, Matthew J Hoke 6, Lindsey M Altenhofen 7, Heather J Painter 7, Jianbing Mu 8, David J P Ferguson 9, Manuel Llinás 7, Rowena E Martin 3, David A Fidock 2 10, Roland A Cooper 6 11, Sanjeev Krishna 1
Affiliations
- PMID: 26420308
- PMCID: PMC4588581
- DOI: 10.1038/srep14552
Mutations in the Plasmodium falciparum chloroquine resistance transporter, PfCRT, enlarge the parasite's food vacuole and alter drug sensitivities
Serena Pulcini et al. Sci Rep. 2015.
Abstract
Mutations in the Plasmodium falciparum chloroquine resistance transporter, PfCRT, are the major determinant of chloroquine resistance in this lethal human malaria parasite. Here, we describe P. falciparum lines subjected to selection by amantadine or blasticidin that carry PfCRT mutations (C101F or L272F), causing the development of enlarged food vacuoles. These parasites also have increased sensitivity to chloroquine and some other quinoline antimalarials, but exhibit no or minimal change in sensitivity to artemisinins, when compared with parental strains. A transgenic parasite line expressing the L272F variant of PfCRT confirmed this increased chloroquine sensitivity and enlarged food vacuole phenotype. Furthermore, the introduction of the C101F or L272F mutation into a chloroquine-resistant variant of PfCRT reduced the ability of this protein to transport chloroquine by approximately 93 and 82%, respectively, when expressed in Xenopus oocytes. These data provide, at least in part, a mechanistic explanation for the increased sensitivity of the mutant parasite lines to chloroquine. Taken together, these findings provide new insights into PfCRT function and PfCRT-mediated drug resistance, as well as the food vacuole, which is an important target of many antimalarial drugs.
Figures
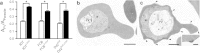
Figure 1. PfCRT mutations.
(a) Schematic representation of PfCRT and positions of previously identified polymorphisms from field isolates (green circles) and from drug-pressured laboratory lines (purple circles). The critical CQ resistance mutation site (K76) is shaded red, and the two residues at which mutations are described in this study are shaded in orange (C101) and blue (L272). (b) PfCRT haplotypes included this study.
Figure 2. Representative morphology of parasite lines FCB_C101F_ and 3D7_L272F_.
(a) Appearance of enlarged FVs in fixed FCB_C101F_ parasites (left panel), when compared with parental FCB parasites of similar developmental stages (right panel). (b) Images of live FCB_C101F_ and FCB trophozoite-stage parasites, using bright-field and dark-field microscopy (left and right panels, respectively). (c) Appearance of enlarged FVs in fixed 3D7_L272F_ parasites (left panel), when compared with parental 3D7 parasites of similar developmental stages (right panel). The diameter of a RBC is ~7 μm.
Figure 3. Morphological comparisons of parasites with mutations in pfcrt compared with their parental controls.
(a) Parasites were synchronized by sorbitol lysis and areas of FVs and parasites (approximately 38 h post-invasion) were measured and expressed as ratios (AFV/AParasite). 3D7, FCB and Dd2Dd2 (open bars; n = 72, 31 and 42, respectively) and 3D7_L272F_, FCBC101F and Dd2Dd2 L272F (closed bars; n = 84, 58 and 88, respectively) parasites were analyzed and significant enlargement of the FV was confirmed in 3D7_L272F_, FCB_C101F_ and Dd2_Dd2 L272F_ parasites relative to 3D7, FCB and Dd2_Dd2_, respectively (*p < 0.0001: two-tailed, unpaired, Student’s t_-test). (b,c) Transmission electron micrographs of 3D7 and 3D7_L272F parasites, respectively, showing the food vacuole (FV) and nucleus (N). Note the enlarged electron lucent FV in 3D7_L272F_ (suggesting changes in the process of hemoglobin degradation and formation of hemozoin crystals). RBCs infected with 3D7_L272F_ displayed approximately 7.5-fold more knobs (arrowheads) on the host surface than 3D7-infected RBCs, although this is likely due to sub-population selection rather than a direct link to the mutation in pfcrt. (insert) Detail of a knob. Bars represent 1 μm (b,c) and 100 nm (insert).
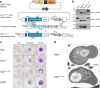
Figure 4. Introduction of PfCRT L272F into Dd2.
(a) Schematic of zinc-finger nuclease (ZFN)-mediated generation of vacuole-enlarged parasites. Dd2 parasites were first enriched for the episomal pcrt_Dd2 L272F_-h_dhfr_ or pcrt_Dd2_-h_dhfr_ donor plasmids (latter not shown). The donor plasmids encoded a cDNA copy of the Dd2 pfcrt allele (dark blue, plasmid), either L272F-mutated (dark blue bump) or wild-type (not shown), followed by a dhfr selection cassette (light grey). Each donor-enriched parasite was then transfected with the pZFN_pfcrt_-bsd plasmid, expressing the genomic (light blue) pfcrt intron 1-targeting ZFN pair (ZFN L and ZFN R, orange) and the bsd selection cassette (dark grey). ZFN-induced recombination in pfcrt yielded either control Dd2_Dd2_ (not shown) or Dd2_Dd2 L272F_ parasites (dark blue, locus). (b) PCR verification of parental, recombinant control, and Dd2_Dd2 L272F_ parasite clones. Primer (p) positions are shown in panel a. (c) Light microscope analysis of representative examples of parental, recombinant control, and experimental parasite clones. Ring morphology for each parasite Dd2, Dd2_Dd2_, Dd2_Dd2 L272F_, and GC03 was normal. Progression through the trophozoite and schizont stages showed normal morphological development except for the Dd2_Dd2 L272F_ clone, which exhibited the characteristic enlarged vacuole and diffuse hemozoin phenotypes seen in 3D7_L272F_ and FCB_C101F_ Giemsa stained parasites. (d) Transmission electron micrographs of Dd2_Dd2_ (i) and Dd2_Dd2 L272F_ parasites (ii) showing similar cytoplasmic appearances except for the enlarged food vacuole (FV) in Dd2_Dd2 L272F_. Note neither parasite exhibits knobs. This confirms similar morphological appearances to those of 3D7 and 3D7_L272F_, respectively but without knob formation. N—Nucleus. Bars represent 1 μm.
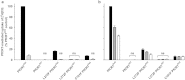
Figure 5. CQ transport activity of the C101F and L272F variants of PfCRT in Xenopus oocytes.
(a,b) The uptake of [3H]CQ into oocytes expressing PfCRT was measured in the absence (closed bars) or presence of 250 μM VP (light grey bars; a), 100 μM BSD (dark grey bars; b), or 500 μM BSD (open bars; b). Within each experiment, measurements were made from 10 oocytes per treatment and uptake was expressed relative to that measured in the PfCRTDd2-expressing oocytes under control conditions. The normalized data obtained from 4–5 separate experiments (each using oocytes from different frogs) were then averaged and are shown + SEM. Both panels show PfCRT-mediated CQ uptake, calculated by subtracting CQ uptake measured in PfCRT_3D7_-expressing oocytes (i.e. the component of CQ accumulation attributable to diffusion; see Supplementary Fig. S3) from that measured in oocytes expressing a variant of PfCRT. In the control treatments, the rates of CQ uptake (pmol/oocyte/h; n = 9 ± SEM) in oocytes expressing PfCRTDd2 and PfCRT3D7 were 23.6 ± 2.3 and 1.3 ± 0.2, respectively. ‘ns’ denotes no significant difference (p > 0.05) in CQ accumulation between oocytes expressing a PfCRT variant (in the presence or absence of VP or BSD) and that measured in the PfCRT3D7-expressing oocytes under control conditions.
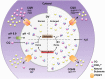
Figure 6. Hypothetical schematic model of the effects of PfCRT mutations.
The FV is acidified by a vacuolar proton pump to create a suitable environment for hemoglobin digestion. The acidic nature of the FV also leads to near complete diprotonation of CQ, which diffuses across the FV membrane in an uncharged form (CQ) and accumulates as a charged form (either CQH+ or CQH22+, although predominantly CQH22+). CQH22+ interferes with the polymerization of toxic heme to non-toxic hemozoin, which leads to parasite death. In normal CQ-sensitive (CQS) parasites, PfCRT, which contains a positive charge in its pore (K76), exports its natural substrates but little, if any, CQH22+. Thus, CQH22+ accumulates in the FV and causes parasite death. In CQ-resistant (CQR) parasites, the positive change in the pore of PfCRT is lost (K76T) and both its natural substrates and CQH22+ are transported out of the FV. As CQH22+ cannot accumulate in the FV, the parasites become resistant to the drug. In 3D7_L272F_ parasites (where the parent strain is already CQS), the mutation may reduce residual transport of CQH22+ out of the FV even further or completely, leading to a greater FV accumulation of CQH22+ and CQ-hypersensitivity or some other mechanism may be responsible for this phenomenon. The mutation also leads to a reduction in the export of natural substrates, resulting in a build-up of these substrates. This causes water to enter the FV by the process of osmosis, leading to swelling. In FCB_C101F_ and Dd2_Dd2 L272F_ parasites (where the parent strains are CQR), the mutations reduce the export of CQH22+ back towards levels measured in CQS lines and also reduce natural substrate export, leading to normal CQ sensitivity and FV swelling, respectively. Note mutations in PfCRT (orange graphic) are denoted by red transmembrane or loop regions, depending on the location of the amino acid change (see Fig. 1a).
Similar articles
- A Variant PfCRT Isoform Can Contribute to Plasmodium falciparum Resistance to the First-Line Partner Drug Piperaquine.
Dhingra SK, Redhi D, Combrinck JM, Yeo T, Okombo J, Henrich PP, Cowell AN, Gupta P, Stegman ML, Hoke JM, Cooper RA, Winzeler E, Mok S, Egan TJ, Fidock DA. Dhingra SK, et al. mBio. 2017 May 9;8(3):e00303-17. doi: 10.1128/mBio.00303-17. mBio. 2017. PMID: 28487425 Free PMC article. - Chloroquine resistance-conferring mutations in pfcrt give rise to a chloroquine-associated H+ leak from the malaria parasite's digestive vacuole.
Lehane AM, Kirk K. Lehane AM, et al. Antimicrob Agents Chemother. 2008 Dec;52(12):4374-80. doi: 10.1128/AAC.00666-08. Epub 2008 Oct 13. Antimicrob Agents Chemother. 2008. PMID: 18852275 Free PMC article. - A Redox-Active Fluorescent pH Indicator for Detecting Plasmodium falciparum Strains with Reduced Responsiveness to Quinoline Antimalarial Drugs.
Jida M, Sanchez CP, Urgin K, Ehrhardt K, Mounien S, Geyer A, Elhabiri M, Lanzer M, Davioud-Charvet E. Jida M, et al. ACS Infect Dis. 2017 Feb 10;3(2):119-131. doi: 10.1021/acsinfecdis.5b00141. Epub 2016 Dec 7. ACS Infect Dis. 2017. PMID: 28183182 - Molecular Mechanisms of Drug Resistance in Plasmodium falciparum Malaria.
Wicht KJ, Mok S, Fidock DA. Wicht KJ, et al. Annu Rev Microbiol. 2020 Sep 8;74:431-454. doi: 10.1146/annurev-micro-020518-115546. Annu Rev Microbiol. 2020. PMID: 32905757 Free PMC article. Review. - PfCRT and its role in antimalarial drug resistance.
Ecker A, Lehane AM, Clain J, Fidock DA. Ecker A, et al. Trends Parasitol. 2012 Nov;28(11):504-14. doi: 10.1016/j.pt.2012.08.002. Epub 2012 Sep 25. Trends Parasitol. 2012. PMID: 23020971 Free PMC article. Review.
Cited by
- How has mass drug administration with dihydroartemisinin-piperaquine impacted molecular markers of drug resistance? A systematic review.
Moss S, Mańko E, Krishna S, Campino S, Clark TG, Last A. Moss S, et al. Malar J. 2022 Jun 11;21(1):186. doi: 10.1186/s12936-022-04181-y. Malar J. 2022. PMID: 35690758 Free PMC article. Review. - Detection of mutations associated with artemisinin resistance at k13-propeller gene and a near complete return of chloroquine susceptible falciparum malaria in Southeast of Tanzania.
Bwire GM, Ngasala B, Mikomangwa WP, Kilonzi M, Kamuhabwa AAR. Bwire GM, et al. Sci Rep. 2020 Feb 26;10(1):3500. doi: 10.1038/s41598-020-60549-7. Sci Rep. 2020. PMID: 32103124 Free PMC article. - Additional PfCRT mutations driven by selective pressure for improved fitness can result in the loss of piperaquine resistance and altered Plasmodium falciparum physiology.
Hagenah LM, Dhingra SK, Small-Saunders JL, Qahash T, Willems A, Schindler KA, Rangel GW, Gil-Iturbe E, Kim J, Akhundova E, Yeo T, Okombo J, Mancia F, Quick M, Roepe PD, Llinás M, Fidock DA. Hagenah LM, et al. mBio. 2024 Jan 16;15(1):e0183223. doi: 10.1128/mbio.01832-23. Epub 2023 Dec 7. mBio. 2024. PMID: 38059639 Free PMC article. - A Variant PfCRT Isoform Can Contribute to Plasmodium falciparum Resistance to the First-Line Partner Drug Piperaquine.
Dhingra SK, Redhi D, Combrinck JM, Yeo T, Okombo J, Henrich PP, Cowell AN, Gupta P, Stegman ML, Hoke JM, Cooper RA, Winzeler E, Mok S, Egan TJ, Fidock DA. Dhingra SK, et al. mBio. 2017 May 9;8(3):e00303-17. doi: 10.1128/mBio.00303-17. mBio. 2017. PMID: 28487425 Free PMC article. - Bibliometric analysis of antimalarial drug resistance.
Zhang J, Shahbaz M, Ijaz M, Zhang H. Zhang J, et al. Front Cell Infect Microbiol. 2024 Feb 12;14:1270060. doi: 10.3389/fcimb.2024.1270060. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38410722 Free PMC article. Review.
References
- Su X., Kirkman L. A., Fujioka H. & Wellems T. E. Complex polymorphisms in an approximately 330 kDa protein are linked to chloroquine-resistant P. falciparum in Southeast Asia and Africa. Cell 91, 593–603 (1997). - PubMed
- Chou A. C., Chevli R. & Fitch C. D. Ferriprotoporphyrin IX fulfills the criteria for identification as the chloroquine receptor of malaria parasites. Biochemistry 19, 1543–1549 (1980). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI050234/AI/NIAID NIH HHS/United States
- T32 AI106711/AI/NIAID NIH HHS/United States
- R01 AI50234/AI/NIAID NIH HHS/United States
- R37 AI050234/AI/NIAID NIH HHS/United States
- R01 AI071121/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources