Why is placentation abnormal in preeclampsia? - PubMed (original) (raw)
Review
Why is placentation abnormal in preeclampsia?
Susan J Fisher. Am J Obstet Gynecol. 2015 Oct.
Abstract
The causes of preeclampsia remain one of the great medical mysteries of our time. This syndrome is thought to occur in 2 stages with abnormal placentation leading to a maternal inflammatory response. Specific regions of the placenta have distinct pathologic features. During normal pregnancy, cytotrophoblasts emigrate from the chorionic villi and invade the uterus, reaching the inner third of the myometrium. This unusual process is made even more exceptional by the fact that the placental cells are hemiallogeneic, coexpressing maternal and paternal genomes. Within the uterine wall, cytotrophoblasts deeply invade the spiral arteries. Cytotrophoblasts migrate up these vessels and replace, in a retrograde fashion, the maternal endothelial lining. They also insert themselves among the smooth muscle cells that form the tunica media. As a result, the spiral arteries attain the physiologic properties that are required to perfuse the placenta adequately. In comparison, invasion of the venous side of the uterine circulation is minimal, sufficient to enable venous return. In preeclampsia, cytotrophoblast invasion of the interstitial uterine compartment is frequently shallow, although not consistently so. In many locations, spiral artery invasion is incomplete. There are many fewer endovascular cytotrophoblasts, and some vessels retain portions of their endothelial lining with relatively intact muscular coats, although others are not modified. Work from our group showed that these defects mirror deficits in the differentiation program that enables cytotrophoblast invasion of the uterine wall. During normal pregnancy, invasion is accompanied by the down-regulation of epithelial-like molecules that are indicative of their ectodermal origin and up-regulation of numerous receptors and ligands that typically are expressed by endothelial or vascular smooth muscle cells. For example, the expression of epithelial-cadherin (the cell-cell adhesion molecule that many ectodermal derivatives use to adhere to one another) becomes nearly undetectable, replaced by vascular-endothelial cadherin, which serves the same purpose in blood vessels. Invading cytotrophoblasts also modulate vascular endothelial growth factor ligands and receptors, at some point in the differentiation process expressing every (mammalian) family member. Molecules in this family play crucial roles in vascular and trophoblast biology, including the prevention of apoptosis. In preeclampsia, this process of vascular mimicry is incomplete, which we theorize hinders the cells interactions with spiral arterioles. What causes these aberrations? Given what is known from animal models and human risk factors, reduced placental perfusion and/or certain disease states (metabolic, immune and cardiovascular) lie upstream. Recent evidence suggests the surprising conclusion that isolation and culture of cytotrophoblasts from the placentas of pregnancies complicated by preeclampsia enables normalization of their gene expression. The affected molecules include SEMA3B, which down-regulates vascular endothelial growth factor signaling through the PI3K/AKT and GSK3 pathways. Thus, some aspects of the aberrant differentiation of cytotrophoblasts within the uterine wall that is observed in situ may be reversible. The next challenge is asking what the instigating causes are. There is added urgency to finding the answers, because these pathways could be valuable therapeutic targets for reversing abnormal placental function in patients.
Keywords: HLA-G; PLGF; angiogenic factor; cytotrophoblast; endoglin; endothelial cell; inflammation; placenta; pregnancy; spiral artery.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Figure 1
Placental cytotrophoblasts invade the uterine wall where they breach veins and extensively remodel maternal spiral arterioles. The bulk of the placenta is composed of numerous tree-like projections termed chorionic villi where maternal-fetal exchange occurs. These structures mediate the passage of nutrients, gases and wastes between fetal blood, which circulates through the villous core, and maternal blood, which circulates through the intervillous space. The uteroplacental circulation is established by cytotrophoblasts that acquire an invasive/endothelial phenotype as they leave the placenta and enter the uterine wall. Differentiation begins when cytotrophoblast progenitors that reside in a single layer surrounding the stromal core of anchoring villi emigrate, forming a cell column. These structures attach to the uterine wall and give rise to cells that invade the underlying decidual stroma. Invasive cytotrophoblasts breach uterine blood vessels connecting both the arterial and the venous circulation to the intervillous space. However, once this connection is made, remodeling of the venous side is halted. By contrast, cytotrophoblasts migrate up the lumina of spiral arterioles, eventually replacing the endothelial lining of the vessels and part of the muscular wall. This process encompasses the decidual and inner third of the myometrial segments of these vessels. NK, natural killer; mϕ, macrophage. Figure and legend reprinted with permission from Development.
Figure 2
VE-cadherin is not detected on cytotrophoblasts (CTBs) in pre-eclamptic placentas. Sections of age-matched 26-wk control (CON, A–D) and HELLP pre-eclamptic (PE, E–H) tissue double stained for cytokeratin (CK; A, C, E, and G) and VE-cadherin (B, D, F, and H). (A, B) Control anchoring villus and placental bed. VE-cadherin staining is not detected on villous CTBs but is present on CTBs within columns and the uterine wall. (C, D) Section of a maternal spiral artery in control placental bed tissue. VE-cadherin staining is especially strong on intravascular CTBs and CTBs that are associated with the vessel wall. (E, F) Section of preeclamptic anchoring villus and placental bed. VE-cadherin staining is not detected. (G, H) Section through a small blood vessel surrounded by CTBs. VE-cadherin is not detected on vessel-associated CTBs or CTBs within the surrounding tissue. However, the endothelial cells (EC) that line the vessel did stain (arrows). Figure and legend reprinted with permission from Journal of Clinical Investigation.
Figure 3
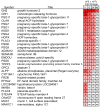
Severe PE-associated aberrations in cytotrophoblast (CTB) gene expression return to control values after 48 hours of culture. RNA was collected immediately after the cells were isolated (0 hour) and after 12, 24, and 48 hours in culture. The relative gene expression levels for CTBs isolated from gestational age matched placentas of patients who delivered due to severe PE (n = 5) relative to noninfected preterm labor (nPTL) are shown as a heat map. The average fold changes for each time point (severe PE vs. nPTL) are shown. ns, no significant difference (LIMMA). Figure and legend reprinted with permission from Journal of Clinical Investigation. The data are a truncated form of the original heatmap that was published.
Similar articles
- Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome.
Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, Alitalo K, Damsky C, Fisher SJ. Zhou Y, et al. Am J Pathol. 2002 Apr;160(4):1405-23. doi: 10.1016/S0002-9440(10)62567-9. Am J Pathol. 2002. PMID: 11943725 Free PMC article. - TGFβ signalling: a nexus between inflammation, placental health and preeclampsia throughout pregnancy.
Horvat Mercnik M, Schliefsteiner C, Sanchez-Duffhues G, Wadsack C. Horvat Mercnik M, et al. Hum Reprod Update. 2024 Jul 1;30(4):442-471. doi: 10.1093/humupd/dmae007. Hum Reprod Update. 2024. PMID: 38519450 Free PMC article. Review. - Up-regulation of CD81 inhibits cytotrophoblast invasion and mediates maternal endothelial cell dysfunction in preeclampsia.
Shen L, Diao Z, Sun HX, Yan GJ, Wang Z, Li RT, Dai Y, Wang J, Li J, Ding H, Zhao G, Zheng M, Xue P, Liu M, Zhou Y, Hu Y. Shen L, et al. Proc Natl Acad Sci U S A. 2017 Feb 21;114(8):1940-1945. doi: 10.1073/pnas.1617601114. Epub 2017 Feb 6. Proc Natl Acad Sci U S A. 2017. PMID: 28167787 Free PMC article. - Failure of physiological transformation and spiral artery atherosis: their roles in preeclampsia.
Staff AC, Fjeldstad HE, Fosheim IK, Moe K, Turowski G, Johnsen GM, Alnaes-Katjavivi P, Sugulle M. Staff AC, et al. Am J Obstet Gynecol. 2022 Feb;226(2S):S895-S906. doi: 10.1016/j.ajog.2020.09.026. Epub 2020 Sep 21. Am J Obstet Gynecol. 2022. PMID: 32971013 Review. - Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion?
Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. Zhou Y, et al. J Clin Invest. 1997 May 1;99(9):2139-51. doi: 10.1172/JCI119387. J Clin Invest. 1997. PMID: 9151786 Free PMC article.
Cited by
- Phosphoproteomics reveals the apoptotic regulation of aspirin in the placenta of preeclampsia-like mice.
Huai J, Li GL, Lin L, Ma JM, Yang HX. Huai J, et al. Am J Transl Res. 2020 Jul 15;12(7):3361-3375. eCollection 2020. Am J Transl Res. 2020. PMID: 32774705 Free PMC article. - Placental development and function in trisomy 21 and mouse models of Down syndrome: Clues for studying mechanisms underlying atypical development.
Adams AD, Guedj F, Bianchi DW. Adams AD, et al. Placenta. 2020 Jan 1;89:58-66. doi: 10.1016/j.placenta.2019.10.002. Epub 2019 Oct 5. Placenta. 2020. PMID: 31683073 Free PMC article. Review. - Involvement of Receptor for Advanced Glycation Endproducts in Hypertensive Disorders of Pregnancy.
Akasaka J, Naruse K, Sado T, Uchiyama T, Makino M, Yamauchi A, Ota H, Sakuramoto-Tsuchida S, Itaya-Hironaka A, Takasawa S, Kobayashi H. Akasaka J, et al. Int J Mol Sci. 2019 Nov 1;20(21):5462. doi: 10.3390/ijms20215462. Int J Mol Sci. 2019. PMID: 31683992 Free PMC article. - Distinct Roles of Classical and Lectin Pathways of Complement in Preeclamptic Placentae.
Belmonte B, Mangogna A, Gulino A, Cancila V, Morello G, Agostinis C, Bulla R, Ricci G, Fraggetta F, Botto M, Garred P, Tedesco F. Belmonte B, et al. Front Immunol. 2022 May 31;13:882298. doi: 10.3389/fimmu.2022.882298. eCollection 2022. Front Immunol. 2022. PMID: 35711467 Free PMC article. - Maternal Serum Placental Growth Factor, Soluble Fms-Like Tyrosine Kinase-1, and Soluble Endoglin in Twin Gestations and the Risk of Preeclampsia-A Systematic Review.
Kosinska-Kaczynska K, Zgliczynska M, Kozlowski S, Wicherek L. Kosinska-Kaczynska K, et al. J Clin Med. 2020 Jan 9;9(1):183. doi: 10.3390/jcm9010183. J Clin Med. 2020. PMID: 31936659 Free PMC article. Review.
References
- Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of pre-eclampsia. The Journal of pathology. 1970;101(4):Pvi. - PubMed
- Genbacev O, Miller RK. Post-implantation differentiation and proliferation of cytotrophoblast cells: in vitro models--a review. Placenta. 2000;21(Suppl A):S45–9. - PubMed
- Zdravkovic T, Genbacev O, Prakobphol A, Cvetkovic M, Schanz A, McMaster M, et al. Nicotine downregulates the l-selectin system that mediates cytotrophoblast emigration from cell columns and attachment to the uterine wall. Reprod Toxicol. 2006;22(1):69–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials