Reconciling Estimates of Cell Proliferation from Stable Isotope Labeling Experiments - PubMed (original) (raw)
. 2015 Oct 5;11(10):e1004355.
doi: 10.1371/journal.pcbi.1004355. eCollection 2015 Oct.
Liset Westera 2, Julia Drylewicz 3, Marjet Elemans 4, Yan Zhang 1, Elizabeth Kelly 1, Rajko Reljic 1, Kiki Tesselaar 2, Rob J de Boer 5, Derek C Macallan 1, José A M Borghans 2, Becca Asquith 4
Affiliations
- PMID: 26437372
- PMCID: PMC4593553
- DOI: 10.1371/journal.pcbi.1004355
Reconciling Estimates of Cell Proliferation from Stable Isotope Labeling Experiments
Raya Ahmed et al. PLoS Comput Biol. 2015.
Abstract
Stable isotope labeling is the state of the art technique for in vivo quantification of lymphocyte kinetics in humans. It has been central to a number of seminal studies, particularly in the context of HIV-1 and leukemia. However, there is a significant discrepancy between lymphocyte proliferation rates estimated in different studies. Notably, deuterated (2)H2-glucose (D2-glucose) labeling studies consistently yield higher estimates of proliferation than deuterated water (D2O) labeling studies. This hampers our understanding of immune function and undermines our confidence in this important technique. Whether these differences are caused by fundamental biochemical differences between the two compounds and/or by methodological differences in the studies is unknown. D2-glucose and D2O labeling experiments have never been performed by the same group under the same experimental conditions; consequently a direct comparison of these two techniques has not been possible. We sought to address this problem. We performed both in vitro and murine in vivo labeling experiments using identical protocols with both D2-glucose and D2O. This showed that intrinsic differences between the two compounds do not cause differences in the proliferation rate estimates, but that estimates made using D2-glucose in vivo were susceptible to difficulties in normalization due to highly variable blood glucose enrichment. Analysis of three published human studies made using D2-glucose and D2O confirmed this problem, particularly in the case of short term D2-glucose labeling. Correcting for these inaccuracies in normalization decreased proliferation rate estimates made using D2-glucose and slightly increased estimates made using D2O; thus bringing the estimates from the two methods significantly closer and highlighting the importance of reliable normalization when using this technique.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
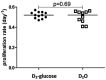
Fig 1. Total CD4+ T cell proliferation rates estimated from D2-glucose and D2O labeling studies in humans.
Each symbol represents a different individual, horizontal lines represent the median of the estimates. (A) Proliferation rates estimated using the kinetic heterogeneity model. (B) Proliferation rates estimated after adjusting for saturation (using the multi-exponential model for the nine-week (9w) D2O and seven-day (7d) D2-glucose labeling, and the kinetic heterogeneity model for one-day (1d) D2-glucose labeling). Although correcting for the saturation of rapidly turning over subpopulations helps to bring the estimates closer (by increasing the proliferation rate estimates obtained in the nine-week D2O labeling study) significant discrepancies remain.
Fig 2. Proliferation rates of Jurkat cells growing in vitro estimated using D2-glucose and D2O labeling.
Each symbol represents the proliferation rate estimated in a separate experiment, horizontal lines represent the median of the estimated proliferation rates.
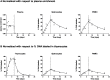
Fig 3. Enrichment curves of D2-glucose labeled mice.
Best fits to the fraction of deuterium enrichment in plasma, and the percentage of label enrichment in the DNA of PBMC, splenocytes and thymocytes after seven-days of D2-glucose labeling. Dots represent individual mice. The end of label administration at day 7 is marked by a dashed vertical line. (A) Conventional normalization: measurements are normalized to the mean plasma enrichment x b g. (B) Normalized with respect to thymocytes: thymocytes were used to determine the maximum percentage of labeled DNA that cells could possibly attain (Methods), and all measured enrichments were scaled to this maximum. The raw data of the % DNA labeled in splenocytes and PBMC is the same in panel A and B but has been normalized using two different approaches.
Fig 4. Enrichment curves of D2O labeled mice.
Best fits to the fraction of deuterium enrichment in plasma (A) and the percentage of label enrichment in the DNA of thymocytes (B), PBMC (C), and splenocytes (D) after seven-days of D2O labeling. Thymocytes were used to determine the maximum percentage of labeled DNA that cells could possibly attain (Methods), and all measured enrichments were scaled to this maximum. Dots represent individual mice. The end of label administration at day 7 is marked by a dashed vertical line.
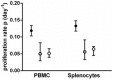
Fig 5. Proliferation rate estimates from 1 week D2-glucose and D2O labeling in mice.
The average proliferation rate of PBMC and splenocytes, obtained by fitting the kinetic heterogenity model to the data collected during labeling with D2-glucose with conventional normalization (using 0.65 × plasma AUC, filled circles), labeling with D2-glucose with normalization to thymocytes (grey-filled circles) and labeling with D2O with conventional normalization i.e. to thymocytes (open circles). Bars represent 95% confidence intervals; AUC = area under the curve.
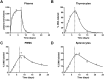
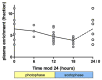
Fig 6. Impact of light-dark cycle on plasma glucose enrichment in mice.
The fraction of deuterium enrichment of plasma glucose is plotted against time since the start of label modulo 24 h. Data from mice housed under reversed day/night condition is translated by 12h so that for all mice 0h represents the start of light phase (8am for normal conditions, 8pm for reversed day/night conditions). Grey circles represent measurements from individual mice, the line connects the medians, the points plotted at 24h/0h are duplicates of the 0h data.
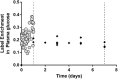
Fig 7. Deuterium enrichment in plasma glucose in one-day and seven-day D2-glucose labeling studies of humans.
The measured enrichment in plasma glucose in the one-day (grey circles) and seven-day (black diamonds) D2-glucose labeling studies. The end of the one-day and seven-day labeling periods are marked by a dashed grey line and a dotted black line respectively. Although subjects in the one-day labeling experiment (N = 8) received approximately twice as much deuterated glucose per day as subjects in the seven-day labeling study (N = 4), their median label enrichment is only slightly higher. Median plasma glucose enrichment in the one-day labeling study = 23%, median plasma glucose enrichment in the seven-day labeling study = 17%.
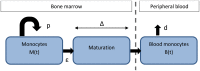
Fig 8. Model of monocyte development.
Monocyte progenitors in the bone marrow (M(t)) proliferate at rate p, transit into a maturation compartment at rate ε, where they mature for a fixed time (Δ) before exiting into the blood compartment (B(t)), from which they disappear at rate d.
Similar articles
- Quantitating lymphocyte homeostasis in vivo in humans using stable isotope tracers.
Westera L, Zhang Y, Tesselaar K, Borghans JA, Macallan DC. Westera L, et al. Methods Mol Biol. 2013;979:107-31. doi: 10.1007/978-1-62703-290-2_10. Methods Mol Biol. 2013. PMID: 23397392 - Measurement of proliferation and disappearance of rapid turnover cell populations in human studies using deuterium-labeled glucose.
Macallan DC, Asquith B, Zhang Y, de Lara C, Ghattas H, Defoiche J, Beverley PC. Macallan DC, et al. Nat Protoc. 2009;4(9):1313-27. doi: 10.1038/nprot.2009.117. Epub 2009 Aug 20. Nat Protoc. 2009. PMID: 19696750 - Explicit kinetic heterogeneity: mathematical models for interpretation of deuterium labeling of heterogeneous cell populations.
Ganusov VV, Borghans JA, De Boer RJ. Ganusov VV, et al. PLoS Comput Biol. 2010 Feb 5;6(2):e1000666. doi: 10.1371/journal.pcbi.1000666. PLoS Comput Biol. 2010. PMID: 20140186 Free PMC article. - Metabolic deuterium oxide (D2O) labeling in quantitative omics studies: A tutorial review.
Kim J, Seo S, Kim TY. Kim J, et al. Anal Chim Acta. 2023 Feb 15;1242:340722. doi: 10.1016/j.aca.2022.340722. Epub 2022 Dec 14. Anal Chim Acta. 2023. PMID: 36657897 Review. - Current best estimates for the average lifespans of mouse and human leukocytes: reviewing two decades of deuterium-labeling experiments.
Borghans JAM, Tesselaar K, de Boer RJ. Borghans JAM, et al. Immunol Rev. 2018 Sep;285(1):233-248. doi: 10.1111/imr.12693. Immunol Rev. 2018. PMID: 30129193 Review.
Cited by
- Estimation methods for heterogeneous cell population models in systems biology.
Waldherr S. Waldherr S. J R Soc Interface. 2018 Oct 31;15(147):20180530. doi: 10.1098/rsif.2018.0530. J R Soc Interface. 2018. PMID: 30381346 Free PMC article. Review. - Deuterated water (2H2O, heavy water) labelling to investigate human cell dynamics in vivo - lessons in protocol design and toxicity from the current literature.
Song A, Zhang Y, Busch R, Asquith B, Macallan D. Song A, et al. Front Immunol. 2025 Apr 28;16:1544193. doi: 10.3389/fimmu.2025.1544193. eCollection 2025. Front Immunol. 2025. PMID: 40356903 Free PMC article. - Current estimates of T cell kinetics in humans.
Macallan DC, Busch R, Asquith B. Macallan DC, et al. Curr Opin Syst Biol. 2019 Dec;18:77-86. doi: 10.1016/j.coisb.2019.10.002. Curr Opin Syst Biol. 2019. PMID: 31922055 Free PMC article. Review. - T cell fate decisions during memory cell generation with aging.
Sturmlechner I, Jain A, Mu Y, Weyand CM, Goronzy JJ. Sturmlechner I, et al. Semin Immunol. 2023 Sep;69:101800. doi: 10.1016/j.smim.2023.101800. Epub 2023 Jul 24. Semin Immunol. 2023. PMID: 37494738 Free PMC article. Review. - How are estimated cellular turnover rates influenced by the dynamics of a source population?
Swain AC, Borghans JAM, de Boer RJ. Swain AC, et al. PLoS Comput Biol. 2025 May 12;21(5):e1013052. doi: 10.1371/journal.pcbi.1013052. eCollection 2025 May. PLoS Comput Biol. 2025. PMID: 40354492 Free PMC article.
References
- Busch R, Neese RA, Awada M, Hayes GM, Hellerstein MK (2007) Measurement of cell proliferation by heavy water labeling. Nat Protoc 2: 3045–3057. - PubMed
- Hellerstein M, Hanley MB, Cesar D, Siler S, Papageorgopoulos C, Wieder E, et al. (1999) Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat Med 5: 83–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 103865/WT_/Wellcome Trust/United Kingdom
- MR/J007439/1/MRC_/Medical Research Council/United Kingdom
- J007439/MRC_/Medical Research Council/United Kingdom
- G0601072/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- G1001052/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources