Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression - PubMed (original) (raw)
Review
Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression
Xin Ye et al. Trends Cell Biol. 2015 Nov.
Abstract
The epithelial-mesenchymal transition (EMT) program has emerged as a central driver of tumor malignancy. Moreover, the recently uncovered link between passage through an EMT and acquisition of stem-like properties indicates that activation of the EMT programs serves as a major mechanism for generating cancer stem cells (CSCs); that is, a subpopulation of cancer cells that are responsible for initiating and propagating the disease. In this review, we summarize the evidence supporting the widespread involvement of the EMT program in tumor pathogenesis and attempt to rationalize the connection between the EMT program and acquisition of stem cell traits. We propose that epithelial-mesenchymal plasticity is likely controlled by multiple varients of the core EMT program, and foresee the need to resolve the various programs and the molecular mechanisms that underlie them.
Keywords: EMT (epithelial–mesenchymal transition); cancer stem cells; plasticity; tumor progression.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
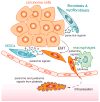
Fig. 1. EMT-inducing signals in the tumor microenvironment
During the process of tumor progression, the EMT program is induced and maintained through concerted actions of a number of stroma-derived paracrine and juxtacrine signals.
Similar articles
- The epithelial-mesenchymal transition and cancer stem cells: functional and mechanistic links.
Liu X, Fan D. Liu X, et al. Curr Pharm Des. 2015;21(10):1279-91. doi: 10.2174/1381612821666141211115611. Curr Pharm Des. 2015. PMID: 25506898 Review. - Involvement of partial EMT in cancer progression.
Saitoh M. Saitoh M. J Biochem. 2018 Oct 1;164(4):257-264. doi: 10.1093/jb/mvy047. J Biochem. 2018. PMID: 29726955 Review. - HIF-1α induces the epithelial-mesenchymal transition in gastric cancer stem cells through the Snail pathway.
Yang SW, Zhang ZG, Hao YX, Zhao YL, Qian F, Shi Y, Li PA, Liu CY, Yu PW. Yang SW, et al. Oncotarget. 2017 Feb 7;8(6):9535-9545. doi: 10.18632/oncotarget.14484. Oncotarget. 2017. PMID: 28076840 Free PMC article. - TGF-β signaling and epithelial-mesenchymal transition in cancer progression.
Katsuno Y, Lamouille S, Derynck R. Katsuno Y, et al. Curr Opin Oncol. 2013 Jan;25(1):76-84. doi: 10.1097/CCO.0b013e32835b6371. Curr Opin Oncol. 2013. PMID: 23197193 Review.
Cited by
- Site-specific metastasis: A cooperation between cancer cells and the metastatic microenvironment.
Izraely S, Witz IP. Izraely S, et al. Int J Cancer. 2021 Mar 15;148(6):1308-1322. doi: 10.1002/ijc.33247. Epub 2020 Aug 27. Int J Cancer. 2021. PMID: 32761606 Free PMC article. Review. - Multifaceted Roles of Long Non-coding RNAs in Head and Neck Cancer.
Duncan L, Shay C, Teng Y. Duncan L, et al. Adv Exp Med Biol. 2021;1286:107-114. doi: 10.1007/978-3-030-55035-6_7. Adv Exp Med Biol. 2021. PMID: 33725348 Free PMC article. Review. - Epithelial-Mesenchymal Transition and Metabolic Switching in Cancer: Lessons From Somatic Cell Reprogramming.
Lai X, Li Q, Wu F, Lin J, Chen J, Zheng H, Guo L. Lai X, et al. Front Cell Dev Biol. 2020 Aug 6;8:760. doi: 10.3389/fcell.2020.00760. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32850862 Free PMC article. Review. - Metabolic and senescence characteristics associated with the immune microenvironment in ovarian cancer.
Xiong J, Fu Y, Huang J, Wang Y, Jin X, Wan X, Huang L, Huang Z. Xiong J, et al. Front Endocrinol (Lausanne). 2023 Nov 22;14:1265525. doi: 10.3389/fendo.2023.1265525. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38075052 Free PMC article. Review. - Age-induced accumulation of methylmalonic acid promotes tumour progression.
Gomes AP, Ilter D, Low V, Endress JE, Fernández-García J, Rosenzweig A, Schild T, Broekaert D, Ahmed A, Planque M, Elia I, Han J, Kinzig C, Mullarky E, Mutvei AP, Asara J, de Cabo R, Cantley LC, Dephoure N, Fendt SM, Blenis J. Gomes AP, et al. Nature. 2020 Sep;585(7824):283-287. doi: 10.1038/s41586-020-2630-0. Epub 2020 Aug 19. Nature. 2020. PMID: 32814897 Free PMC article.
References
- Akalay I, Janji B, Hasmim M, Noman MZ, Andre F, De Cremoux P, Bertheau P, Badoual C, Vielh P, Larsen AK, et al. Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T-cell-mediated lysis. Cancer Res. 2013;73:2418–2427. - PubMed
- Akalay I, Tan TZ, Kumar P, Janji B, Mami-Chouaib F, Charpy C, Vielh P, Larsen AK, Thiery JP, Sabbah M, et al. Targeting WNT1-inducible signaling pathway protein 2 alters human breast cancer cell susceptibility to specific lysis through regulation of KLF-4 and miR-7 expression. Oncogene. 2015;34:2261–2271. - PubMed
- Aleskandarany MA, Negm OH, Green AR, Ahmed MA, Nolan CC, Tighe PJ, Ellis IO, Rakha EA. Epithelial mesenchymal transition in early invasive breast cancer: an immunohistochemical and reverse phase protein array study. Breast Cancer Res Treat. 2014;145:339–348. - PubMed
Publication types
MeSH terms
Grants and funding
- R01-CA078461/CA/NCI NIH HHS/United States
- K99-CA194160/CA/NCI NIH HHS/United States
- P01 CA080111/CA/NCI NIH HHS/United States
- U01-CA184897/CA/NCI NIH HHS/United States
- U01 CA184897/CA/NCI NIH HHS/United States
- P01-CA080111/CA/NCI NIH HHS/United States
- R01 CA078461/CA/NCI NIH HHS/United States
- K99 CA194160/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources