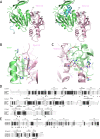Insights into molecular plasticity in protein complexes from Trm9-Trm112 tRNA modifying enzyme crystal structure - PubMed (original) (raw)
. 2015 Dec 15;43(22):10989-1002.
doi: 10.1093/nar/gkv1009. Epub 2015 Oct 4.
Affiliations
- PMID: 26438534
- PMCID: PMC4678810
- DOI: 10.1093/nar/gkv1009
Insights into molecular plasticity in protein complexes from Trm9-Trm112 tRNA modifying enzyme crystal structure
Juliette Létoquart et al. Nucleic Acids Res. 2015.
Abstract
Most of the factors involved in translation (tRNA, rRNA and proteins) are subject to post-transcriptional and post-translational modifications, which participate in the fine-tuning and tight control of ribosome and protein synthesis processes. In eukaryotes, Trm112 acts as an obligate activating platform for at least four methyltransferases (MTase) involved in the modification of 18S rRNA (Bud23), tRNA (Trm9 and Trm11) and translation termination factor eRF1 (Mtq2). Trm112 is then at a nexus between ribosome synthesis and function. Here, we present a structure-function analysis of the Trm9-Trm112 complex, which is involved in the 5-methoxycarbonylmethyluridine (mcm(5)U) modification of the tRNA anticodon wobble position and hence promotes translational fidelity. We also compare the known crystal structures of various Trm112-MTase complexes, highlighting the structural plasticity allowing Trm112 to interact through a very similar mode with its MTase partners, although those share less than 20% sequence identity.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
Figure 1.
Structure of the _Yl_Trm9-Trm112 complex. (A) Ribbon representations of the crystal structure of the _Yl_Trm9N38-Trm112 complex (left) and of the model of the _Yl_Trm9N20-Trm112 complex (right). On the left panel, residues 39–44, which adopt different conformations between our crystal structure and our model, are colored in blue. On the right panel, the modeled _Yl_Trm9 fragment encompassing residues 20–39 (helix αY) is shown in blue. The modeled SAM molecule is shown in sticks. _Yl_Trm112 secondary structure elements are labeled in italics. (B and C) Detailed views of the residues involved in Yl_Trm9N38-Trm112 interface. Hydrogen bonds and salt bridges are show by black dotted lines. (D) Sequence alignments of Trm9 orthologs from Y. lipolytica (Yl_Trm9), Homo sapiens (ABH8) and S. cerevisiae (_Sc_Trm9). Strictly conserved residues are in white on a black background. Partially conserved amino acids are boxed. Secondary structure elements assigned from the _Yl_Trm9N38 crystal structure are indicated above the alignment. Black stars indicate residues involved in complex formation. Filled or open circles below the alignment indicate residues, which mutation into Ala results in zymocin resistance or sensitivity, respectively. For clarity, both _Yl_Trm9 and _Sc_Trm9 numbering are indicated. Two regions corresponding to fragments present only in _Sc_Trm9 or ABH8 have been omitted. (E) Sequence alignments of Trm112 orthologs from Y. lipolytica, Homo sapiens and S. cerevisiae. Strictly conserved residues are in white on a black background. Partially conserved amino acids are boxed. Secondary structure elements assigned from the _Yl_Trm112 crystal structure are indicated above the alignment. Black stars indicate residues involved in complex formation. Panels D and E were generated using the ESPript server (49).
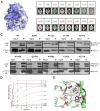
Figure 2.
Trm9 active site mapping. (A) Mapping of the sequence conservation score at the surface of the _Yl_Trm9-Trm112 model. Coloring is from gray (low conservation) to blue (highly conserved). The conservation score was calculated using the CONSURF server (50). (B) Analyses of the zymocin phenotype of yeast mutant strains by eclipse assay. Each S. cerevisiae mutated strain was subjected to killer eclipse assay with the Kluyveromyces lactis AWJ137 killer strain (top) and the Kluyveromyces lactis NK40 non-killer strain (as a control, bottom). The presence or absence of an eclipse around the killer strain shows the sensitivity or resistance of the mutated strain to zymocin toxin, respectively. Resistant and sensitive strains are labeled in red and green, respectively. (C) Effect of Trm9 mutations on _Sc_Trm9/_Sc_Trm112 in vivo interaction. Soluble protein extracts (Input: 1/50th of total proteins, i.e. 10 μg) and immunoprecipitates (IP: 1/10th of immunoprecipitated material) were subjected to 15% SDS–PAGE analysis and immunoblotted using mouse anti-Myc (Trm9–13Myc) or rabbit anti-Trm112 as primary antibodies and sheep anti-mouse or sheep anti-rabbit HRP-conjugated IgG as secondary antibodies, respectively. Note that for the anti-myc probing, the lower bands observed in some of the input lanes result from Trm9-myc protein degradation whereas the band observed in the IP lanes comes from mouse primary antibody heavy chain cross-reacting with anti-mouse secondary antibody. IP were performed using 9E10 anti-myc monoclonal antibodies. (D) Enzymatic activity of Trm112-Trm9 mutants. The curves obtained after fitting of the experimental data with equation given in the Materials and Methods section are shown by lines using the same color code as for the symbols. (E) Detailed representation of the _Yl_Trm9 active site. Side chains from residues which substitution by Ala strongly (brown) or only weakly (green) affect enzymatic activity are shown as sticks. For clarity, _Sc_Trm9 numbering is used. A manually docked cm5U nucleotide is shown as beige sticks and potential hydrogen bonds that it could form with Trm9 active site residues are depicted by dashed black lines. The modeled SAM molecule is shown as blue sticks and the methyl group to be transferred is shown as a sphere.
Figure 3.
Structural comparison of Trm112-MTase complexes. (A) Structure-based sequence alignment of MTases interacting with Trm112. Only regions of these MTases that interact with Trm112 are shown. Residues directly contacting Trm112 in the X-ray structures of the complexes are in white on a black background. Residues from _Sc_Trm9, _Sc_Trm11 and _Sc_Mtq2, matching with interface residues from _Yl_Trm9 and _Ec_Mtq2, are shown in black on a yellow background. _Yl_Trm9 secondary structure elements are depicted below the alignment. Stars below the alignment indicate residues strictly conserved in all 4 _Sc_MTases considered. Residues highlighted in panels C to E are boxed in red. (B) Superimposition of the structures of the _Yl_Trm9-Trm112, _Sc_Bud23-Trm112 and _Ec_Mtq2-Trm112 complexes. (C–E) Detailed comparison of the hydrophobic core (C) and electrostatic hot spots (D and E) involved in _Yl_Trm9-Trm112 and _Sc_Bud23-Trm112 complexes. Same color code as panel B. (F) Effect of ectopic plasmid-driven expression of _Yl_Trm112 (+pYlTrm112) and _Sc_Trm112 (+pScTrm112) proteins on the susceptibility to zymocin of S. cerevisiae expressing either _Sc_Trm9 (dark gray) or _Yl_Trm9 (light gray) WT protein from the unique genomic copy of the corresponding gene under the control of ScTRM9 natural promoter.
Similar articles
- Activation mode of the eukaryotic m2G10 tRNA methyltransferase Trm11 by its partner protein Trm112.
Bourgeois G, Marcoux J, Saliou JM, Cianférani S, Graille M. Bourgeois G, et al. Nucleic Acids Res. 2017 Feb 28;45(4):1971-1982. doi: 10.1093/nar/gkw1271. Nucleic Acids Res. 2017. PMID: 27986851 Free PMC article. - Trm112, a Protein Activator of Methyltransferases Modifying Actors of the Eukaryotic Translational Apparatus.
Bourgeois G, Létoquart J, van Tran N, Graille M. Bourgeois G, et al. Biomolecules. 2017 Jan 27;7(1):7. doi: 10.3390/biom7010007. Biomolecules. 2017. PMID: 28134793 Free PMC article. Review. - tRNA and protein methylase complexes mediate zymocin toxicity in yeast.
Studte P, Zink S, Jablonowski D, Bär C, von der Haar T, Tuite MF, Schaffrath R. Studte P, et al. Mol Microbiol. 2008 Sep;69(5):1266-77. doi: 10.1111/j.1365-2958.2008.06358.x. Epub 2008 Jul 24. Mol Microbiol. 2008. PMID: 18657261 - Production of yeast (m2G10) methyltransferase (Trm11 and Trm112 complex) in a wheat germ cell-free translation system.
Okada K, Muneyoshi Y, Endo Y, Hori H. Okada K, et al. Nucleic Acids Symp Ser (Oxf). 2009;(53):303-4. doi: 10.1093/nass/nrp152. Nucleic Acids Symp Ser (Oxf). 2009. PMID: 19749381 - Two-subunit enzymes involved in eukaryotic post-transcriptional tRNA modification.
Guy MP, Phizicky EM. Guy MP, et al. RNA Biol. 2014;11(12):1608-18. doi: 10.1080/15476286.2015.1008360. RNA Biol. 2014. PMID: 25625329 Free PMC article. Review.
Cited by
- Urm1: A Non-Canonical UBL.
Termathe M, Leidel SA. Termathe M, et al. Biomolecules. 2021 Jan 22;11(2):139. doi: 10.3390/biom11020139. Biomolecules. 2021. PMID: 33499055 Free PMC article. Review. - Human TRMT112-Methyltransferase Network Consists of Seven Partners Interacting with a Common Co-Factor.
Brūmele B, Mutso M, Telanne L, Õunap K, Spunde K, Abroi A, Kurg R. Brūmele B, et al. Int J Mol Sci. 2021 Dec 18;22(24):13593. doi: 10.3390/ijms222413593. Int J Mol Sci. 2021. PMID: 34948388 Free PMC article. - Roles of Elongator Dependent tRNA Modification Pathways in Neurodegeneration and Cancer.
Hawer H, Hammermeister A, Ravichandran KE, Glatt S, Schaffrath R, Klassen R. Hawer H, et al. Genes (Basel). 2018 Dec 28;10(1):19. doi: 10.3390/genes10010019. Genes (Basel). 2018. PMID: 30597914 Free PMC article. Review. - The occurrence order and cross-talk of different tRNA modifications.
Li J, Zhu WY, Yang WQ, Li CT, Liu RJ. Li J, et al. Sci China Life Sci. 2021 Sep;64(9):1423-1436. doi: 10.1007/s11427-020-1906-4. Epub 2021 Apr 19. Sci China Life Sci. 2021. PMID: 33881742 Review. - Expanded tRNA methyltransferase family member TRMT9B regulates synaptic growth and function.
Hogan CA, Gratz SJ, Dumouchel JL, Thakur RS, Delgado A, Lentini JM, Madhwani KR, Fu D, O'Connor-Giles KM. Hogan CA, et al. EMBO Rep. 2023 Oct 9;24(10):e56808. doi: 10.15252/embr.202356808. Epub 2023 Aug 29. EMBO Rep. 2023. PMID: 37642556 Free PMC article.
References
- Hopper A.K., Phizicky E.M. tRNA transfers to the limelight. Genes Dev. 2003;17:162–180. - PubMed
- Johansson M.J.O., Bystrom A.S. In: Fine-Tuning of RNA Functions by Modification and Editing. Grosjean H, editor. Heidelberg: Springer-Verlag; 2005. pp. 87–119.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases