The Nuclear Proteome of a Vertebrate - PubMed (original) (raw)
. 2015 Oct 19;25(20):2663-71.
doi: 10.1016/j.cub.2015.08.047. Epub 2015 Oct 1.
Thomas Güttler 2, Leonid Peshkin 3, Graeme C McAlister 2, Matthew Sonnett 1, Keisuke Ishihara 3, Aaron C Groen 3, Marc Presler 3, Brian K Erickson 2, Timothy J Mitchison 3, Marc W Kirschner 4, Steven P Gygi 5
Affiliations
- PMID: 26441354
- PMCID: PMC4618192
- DOI: 10.1016/j.cub.2015.08.047
The Nuclear Proteome of a Vertebrate
Martin Wühr et al. Curr Biol. 2015.
Abstract
The composition of the nucleoplasm determines the behavior of key processes such as transcription, yet there is still no reliable and quantitative resource of nuclear proteins. Furthermore, it is still unclear how the distinct nuclear and cytoplasmic compositions are maintained. To describe the nuclear proteome quantitatively, we isolated the large nuclei of frog oocytes via microdissection and measured the nucleocytoplasmic partitioning of ∼9,000 proteins by mass spectrometry. Most proteins localize entirely to either nucleus or cytoplasm; only ∼17% partition equally. A protein's native size in a complex, but not polypeptide molecular weight, is predictive of localization: partitioned proteins exhibit native sizes larger than ∼100 kDa, whereas natively smaller proteins are equidistributed. To evaluate the role of nuclear export in maintaining localization, we inhibited Exportin 1. This resulted in the expected re-localization of proteins toward the nucleus, but only 3% of the proteome was affected. Thus, complex assembly and passive retention, rather than continuous active transport, is the dominant mechanism for the maintenance of nuclear and cytoplasmic proteomes.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
Figure 1
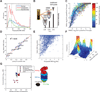
Quantification of nucleocytoplasmic partitioning of the X. laevis oocyte proteome. A) Oocytes were dissected manually in three replicates, proteins digested, TMT-labeled and analyzed separately, with two different methods of accurate quantitative proteomics (MultiNotch- MS3 and TMTC). B) The Relative Nuclear Concentration (RNC) was determined for 9262 proteins. The replicates correlated with an R2 of at least 0.94. C) RNC histogram of all quantified proteins. D) Histogram of RNC values for proteins matched with the human MitoCarta database. E) RNC histogram for proteins classified as nuclear within four commonly used subcellular localization databases are highly enriched for truly nuclear proteins (purple). However, the individual databases show only moderate agreement among themselves and with our data.
Figure 2
Correlation of molecular weight and nucleocytoplasmic partitioning. A) Polypeptide MW is not a strong determinant of nucleocytoplasmic distribution. B) To estimate native protein sizes, cell lysate was percolated through filters of 30 or 100 kDa MW cutoff, respectively. The proteins’ relative passage was quantified with the MultiNotch-MS3 approach. C) Ratios of input and flow-through of the indicated filters were plotted and fitted with a spline. Color code and data point size indicate polypeptide MW. Data point projection onto the spline yielded a “proxy for protein size”, ranging from 0 (small – bottom left) to 1 (large – top right). D) This “proxy for protein size” and the experimentally determined native MW for various vertebrate proteins correlate with an R2 of 0.95. This relationship allowed us to regress the native proteins size in a proteome-wide fashion. E) Plot of native MW versus polypeptide MW indicates that many proteins behave significantly larger than their polypeptide MW suggests. The few proteins for which we measured smaller native MW than polypeptide MW likely represent measurement errors. F) Histogram relating native MW and RNC. Proteins smaller than ~100 kDa are preferentially equipartitioned whereas partitioned proteins are typically larger. However, a subset of natively large proteins is close to equipartitioned. Among them we found the proteasome and APC/C. G) Plot of estimated concentrations and RNCs for the subunits of the proteasome and the APC/C. Interestingly, the 19S and 11S α, β caps are slightly more nuclear than the core proteasome. In contrast, the 11S γ cap is exclusively nuclear.
Figure 3
Nucleocytoplasmic protein partitioning upon inhibition of Exportin 1. A) Experimental setup to determine the change of RNCs upon inhibition of Exportin 1 with LMB. B) RNCs determined for control oocytes and oocytes treated with LMB (24 h) were plotted (experiment 1). The majority of proteins did not change its localization significantly (97%). Three proteins, which re-localized to the nucleus, are highlighted for illustration. C) Scatter plot of RNC changes after 24h in LMB for (experiments 1 and 2). Under the assumption of noise being symmetric and LMB causing nuclear, but not cytoplasmic re-localization, we could estimate the false discovery rate (FDR) of LMB responders. With an FDR cutoff of ~1% (dotted lines) we detected 226 confident LMB responders. D) RNCs for all time points and replicates for the three highlighted proteins. E) Most subunits of the APC/C responded to LMB, suggesting that at least some large complexes present in nucleus and cytoplasm (Fig. 2F) are equipartitioned via active bidirectional transport. We did not see any evidence for Exportin 1-dependent nuclear transport of the proteasome. F) Kinases are overrepresented among LMB responders (p-value = 0.002). The diagram shows these kinases.
Figure 4
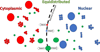
The maintenance of nucleocytoplasmic partitioning is dominated by passive retention. Nuclear pores (depicted as holes in the nuclear envelope) permit the passage of small molecules but restrict that of larger ones. We observed that the vast majority of proteins smaller than ~100 kDa (small green circles) have similar concentrations in nucleus and cytoplasm. Diffusion through nuclear pores allows these proteins to equilibrate between nucleus and cytoplasm. Nearly all partitioned proteins (red or blue) have a native molecular weight larger than ~100 kDa, which prevents efficient diffusion through nuclear pores. Only very few natively small proteins are partitioned via continuous active transport. We also find a subset of natively large but equipartitioned proteins (large green circles). For some of these we provide evidence that they are equilibrated by active bidirectional transport.
Similar articles
- Proteomics of nucleocytoplasmic partitioning.
Nguyen T, Pappireddi N, Wühr M. Nguyen T, et al. Curr Opin Chem Biol. 2019 Feb;48:55-63. doi: 10.1016/j.cbpa.2018.10.027. Epub 2018 Nov 23. Curr Opin Chem Biol. 2019. PMID: 30472625 Free PMC article. Review. - Nucleocytoplasmic shuttling of the thyroid hormone receptor alpha.
Bunn CF, Neidig JA, Freidinger KE, Stankiewicz TA, Weaver BS, McGrew J, Allison LA. Bunn CF, et al. Mol Endocrinol. 2001 Apr;15(4):512-33. doi: 10.1210/mend.15.4.0619. Mol Endocrinol. 2001. PMID: 11266504 - Use of intact Xenopus oocytes in nucleocytoplasmic transport studies.
Panté N. Panté N. Methods Mol Biol. 2006;322:301-14. doi: 10.1007/978-1-59745-000-3_21. Methods Mol Biol. 2006. PMID: 16739732 Review. - Use of Xenopus laevis oocyte nuclei and nuclear envelopes in nucleocytoplasmic transport studies.
Peters R. Peters R. Methods Mol Biol. 2006;322:259-72. doi: 10.1007/978-1-59745-000-3_18. Methods Mol Biol. 2006. PMID: 16739729 Review. - Characterization of RanBPM molecular determinants that control its subcellular localization.
Salemi LM, Loureiro SO, Schild-Poulter C. Salemi LM, et al. PLoS One. 2015 Feb 6;10(2):e0117655. doi: 10.1371/journal.pone.0117655. eCollection 2015. PLoS One. 2015. PMID: 25659156 Free PMC article.
Cited by
- Mechanism of exportin retention in the cell nucleus.
Kapinos LE, Kalita J, Kassianidou E, Rencurel C, Lim RYH. Kapinos LE, et al. J Cell Biol. 2024 Feb 5;223(2):e202306094. doi: 10.1083/jcb.202306094. Epub 2024 Jan 19. J Cell Biol. 2024. PMID: 38241019 Free PMC article. - Control of zygotic genome activation in Xenopus.
Blitz IL, Cho KWY. Blitz IL, et al. Curr Top Dev Biol. 2021;145:167-204. doi: 10.1016/bs.ctdb.2021.03.003. Epub 2021 Apr 19. Curr Top Dev Biol. 2021. PMID: 34074529 Free PMC article. Review. - Determining protein polarization proteome-wide using physical dissection of individual Stentor coeruleus cells.
Lin A, Piehowski PD, Tsai CF, Makushok T, Yi L, Diaz U, Yan C, Summers D, Sood P, Smith RD, Liu T, Marshall WF. Lin A, et al. Curr Biol. 2022 May 23;32(10):2300-2308.e4. doi: 10.1016/j.cub.2022.03.078. Epub 2022 Apr 20. Curr Biol. 2022. PMID: 35447087 Free PMC article. - Synthetic 10FN3-based mono- and bivalent inhibitors of MDM2/X function.
Lau SY, Siau JW, Sobota RM, Wang CI, Zhong P, Lane DP, Ghadessy FJ. Lau SY, et al. Protein Eng Des Sel. 2018 Jul 1;31(7-8):301-312. doi: 10.1093/protein/gzy018. Protein Eng Des Sel. 2018. PMID: 30169723 Free PMC article. - A quantitative model of developmental RTK signaling.
Goyal Y, Schüpbach T, Shvartsman SY. Goyal Y, et al. Dev Biol. 2018 Oct 1;442(1):80-86. doi: 10.1016/j.ydbio.2018.07.012. Epub 2018 Jul 17. Dev Biol. 2018. PMID: 30026122 Free PMC article. Review.
References
- Poon IK, Jans DA. Regulation of nuclear transport: central role in development and transformation? Traffic. 2005;6:173–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM095450/GM/NIGMS NIH HHS/United States
- R01 HD073104/HD/NICHD NIH HHS/United States
- R01 GM039565/GM/NIGMS NIH HHS/United States
- T32 GM095450/GM/NIGMS NIH HHS/United States
- R01 GM103785/GM/NIGMS NIH HHS/United States
- R01GM39565/GM/NIGMS NIH HHS/United States
- R01HD073104/HD/NICHD NIH HHS/United States
- R01GM103785/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials