The aged brain: genesis and fate of residual progenitor cells in the subventricular zone - PubMed (original) (raw)
Review
The aged brain: genesis and fate of residual progenitor cells in the subventricular zone
Vivian Capilla-Gonzalez et al. Front Cell Neurosci. 2015.
Abstract
Neural stem cells (NSCs) persist in the adult mammalian brain through life. The subventricular zone (SVZ) is the largest source of stem cells in the nervous system, and continuously generates new neuronal and glial cells involved in brain regeneration. During aging, the germinal potential of the SVZ suffers a widespread decline, but the causes of this turn down are not fully understood. This review provides a compilation of the current knowledge about the age-related changes in the NSC population, as well as the fate of the newly generated cells in the aged brain. It is known that the neurogenic capacity is clearly disrupted during aging, while the production of oligodendroglial cells is not compromised. Interestingly, the human brain seems to primarily preserve the ability to produce new oligodendrocytes instead of neurons, which could be related to the development of neurological disorders. Further studies in this matter are required to improve our understanding and the current strategies for fighting neurological diseases associated with senescence.
Keywords: aging; cell migration; neural stem cells; neurogenesis; oligodendrogenesis; rostral migratory stream; subventricular zone.
Figures
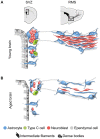
Figure 1
Schematic representation of the subventricular zone (SVZ) and rostral migratory stream (RMS) in the young and aged rodent brain. (A) In the young brain, ependymal cells with cubical morphology integrate the barrier that separates the SVZ neurogenic cells from the lateral ventricle. Neuroblasts form large chains ensheathed by gliotubes of astrocytes. Thus, neuroblasts migrate through these migratory structures, which emerge from the SVZ and coalesce into the RMS that ends in the olfactory bulb (OB). (B) During aging, ependymal cells are flattened and their cilia scatter. Both ependymal cells and astrocytes accumulate dense bodies and intermediate filaments in their cytoplasm. There is a decrease in the number of neural stem cells (NSCs) identified as astrocytes contacting the ventricle, intermediate progenitor cells, and neuroblasts. As a result, the RMS tends to disappear in the aged brain.
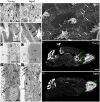
Figure 2
Age-related changes in the ultrastructure of the neurogenic niches. (A,A′) Astrocytes accumulate dense bodies (box) in their cytoplasm during aging. Scale bar: 2 micra. (B,B′) Detail of intermediate filaments (arrows) in astrocytic cells. Note that they are more abundant in aged cells. Scale bar: 500 nm. (C,C′) Detail of lipid droplets in ependymal cells, displaying a larger size during aging. Scale bar: 5 micra. (D,D′) Ependymal cells are flattened in the aged brain, resulting in large gaps between ciliary tufts (arrows). Scale bar: 2 micra. (E) Under scanning electron microscopy, whole-mount preparation of the lateral ventricle shows a deep network of axons (arrows) in the aged brain. Scale bar: 5 micra. (F) DAPI (4′,6-diamidino-2-phenylindole) fluorescent staining shows a remarkable RMS (arrows) from the lateral ventricle to the OB in the young brain. Scale bar: 1 mm. (G) Conversely, the RMS is not evident in the aged brain. Scale bar: 1 mm. b, astrocyte; e, ependymal cell; Cb, cerebellum; Ctx, cerebral cortex; Lp, lipid droplets; Lv, lateral ventricle; OB, olfactory bulb. Images (F,G) have been adapted with permission from Capilla-Gonzalez et al. (2013).
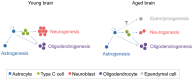
Figure 3
Schematic representation of the fate of newly generated cells in the young and aged SVZ. In the young SVZ, an important number of NSCs differentiate into neurons, while they generate oligodendrocytes and astrocytes to a lesser extent. Aging alters the balance between neurogenesis and gliogenesis. As consequence, neurogenesis is reduced in the aged SVZ, while oligodrendrogenesis is maintained. It is still under debate whether ependymogenesis occurs in the aged SVZ.
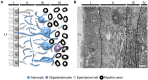
Figure 4
Organization of the adult human SVZ. (A) Diagram representing the adult human SVZ. A monolayer of ependymal cells (Layer I) separates the lateral ventricle from the SVZ. Adjacent to it, a gap or hypocellular layer is mostly composed of GFAP+ cellular expansions (Layer II). Next to the gap layer, the astrocyte ribbon is represented (Layer III), continued by a transition zone to the brain parenchyma (Layer IV). (B) Electron microscopy coronal image of the human SVZ obtained from a 53-year-old female donor. Note the typical organization of this human neurogenic niche (Layers I to IV). b, astrocyte; e, ependymal cell; m, microglia. Scale bar: 4 μm.
Similar articles
- The generation of oligodendroglial cells is preserved in the rostral migratory stream during aging.
Capilla-Gonzalez V, Cebrian-Silla A, Guerrero-Cazares H, Garcia-Verdugo JM, Quiñones-Hinojosa A. Capilla-Gonzalez V, et al. Front Cell Neurosci. 2013 Sep 11;7:147. doi: 10.3389/fncel.2013.00147. eCollection 2013. Front Cell Neurosci. 2013. PMID: 24062640 Free PMC article. - Grafted Subventricular Zone Neural Stem Cells Display Robust Engraftment and Similar Differentiation Properties and Form New Neurogenic Niches in the Young and Aged Hippocampus.
Shetty AK, Hattiangady B. Shetty AK, et al. Stem Cells Transl Med. 2016 Sep;5(9):1204-15. doi: 10.5966/sctm.2015-0270. Epub 2016 May 18. Stem Cells Transl Med. 2016. PMID: 27194744 Free PMC article. - Persistent Cyfip1 Expression Is Required to Maintain the Adult Subventricular Zone Neurogenic Niche.
Habela CW, Yoon KJ, Kim NS, Taga A, Bell K, Bergles DE, Maragakis NJ, Ming GL, Song H. Habela CW, et al. J Neurosci. 2020 Mar 4;40(10):2015-2024. doi: 10.1523/JNEUROSCI.2249-19.2020. Epub 2020 Jan 27. J Neurosci. 2020. PMID: 31988061 Free PMC article. - Mosaic Subventricular Origins of Forebrain Oligodendrogenesis.
Azim K, Berninger B, Raineteau O. Azim K, et al. Front Neurosci. 2016 Mar 24;10:107. doi: 10.3389/fnins.2016.00107. eCollection 2016. Front Neurosci. 2016. PMID: 27047329 Free PMC article. Review. - The heterogeneity of adult neural stem cells and the emerging complexity of their niche.
Alvarez-Buylla A, Kohwi M, Nguyen TM, Merkle FT. Alvarez-Buylla A, et al. Cold Spring Harb Symp Quant Biol. 2008;73:357-65. doi: 10.1101/sqb.2008.73.019. Epub 2008 Nov 6. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19022766 Review.
Cited by
- Primary cilia in Parkinson's disease: summative roles in signaling pathways, genes, defective mitochondrial function, and substantia nigra dopaminergic neurons.
Tian Z, Zhang Y, Xu J, Yang Q, Hu D, Feng J, Gai C. Tian Z, et al. Front Aging Neurosci. 2024 Sep 18;16:1451655. doi: 10.3389/fnagi.2024.1451655. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39364348 Free PMC article. Review. - A core scientific problem in the treatment of central nervous system diseases: newborn neurons.
Hao P, Yang Z, So KF, Li X. Hao P, et al. Neural Regen Res. 2024 Dec 1;19(12):2588-2601. doi: 10.4103/NRR.NRR-D-23-01775. Epub 2024 Apr 1. Neural Regen Res. 2024. PMID: 38595278 Free PMC article. - Exploring the Intricacies of Neurogenic Niches: Unraveling the Anatomy and Neural Microenvironments.
Sánchez-Gomar I, Geribaldi-Doldán N, Santos-Rosendo C, Sanguino-Caneva C, Carrillo-Chapman C, Fiorillo-Moreno O, Villareal Camacho JL, Quiroz EN, Verástegui C. Sánchez-Gomar I, et al. Biomolecules. 2024 Mar 12;14(3):335. doi: 10.3390/biom14030335. Biomolecules. 2024. PMID: 38540755 Free PMC article. Review. - Hericium erinaceus Extract Exerts Beneficial Effects on Gut-Neuroinflammaging-Cognitive Axis in Elderly Mice.
Priori EC, Ratto D, De Luca F, Sandionigi A, Savino E, Giammello F, Romeo M, Brandalise F, Roda E, Rossi P. Priori EC, et al. Biology (Basel). 2023 Dec 28;13(1):18. doi: 10.3390/biology13010018. Biology (Basel). 2023. PMID: 38248449 Free PMC article. - Ganglioside GD3 regulates neural stem cell quiescence and controls postnatal neurogenesis.
Fuchigami T, Itokazu Y, Yu RK. Fuchigami T, et al. Glia. 2024 Jan;72(1):167-183. doi: 10.1002/glia.24468. Epub 2023 Sep 5. Glia. 2024. PMID: 37667994
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources