The Effect of Changes in Cardiorespiratory Fitness and Weight on Obstructive Sleep Apnea Severity in Overweight Adults with Type 2 Diabetes - PubMed (original) (raw)
Randomized Controlled Trial
. 2016 Feb 1;39(2):317-25.
doi: 10.5665/sleep.5436.
David M Reboussin 2, Gary D Foster 3 4, Thomas B Rice 1, Elsa S Strotmeyer 1, John M Jakicic 1, Richard P Millman 5, F Xavier Pi-Sunyer 6, Anne B Newman 1, Thomas A Wadden 7, Gary Zammit 8, Samuel T Kuna 7 9; Sleep AHEAD Research Group of the Look AHEAD Research Group
Collaborators, Affiliations
- PMID: 26446118
- PMCID: PMC4712401
- DOI: 10.5665/sleep.5436
Randomized Controlled Trial
The Effect of Changes in Cardiorespiratory Fitness and Weight on Obstructive Sleep Apnea Severity in Overweight Adults with Type 2 Diabetes
Christopher E Kline et al. Sleep. 2016.
Abstract
Study objectives: To examine the effect of changes in cardiorespiratory fitness on obstructive sleep apnea (OSA) severity prior to and following adjustment for changes in weight over the course of a 4-y weight loss intervention.
Methods: As secondary analyses of a randomized controlled trial, 263 overweight/obese adults with type 2 diabetes and OSA participated in an intensive lifestyle intervention or education control condition. Measures of OSA severity, cardiorespiratory fitness, and body weight were obtained at baseline, year 1, and year 4. Change in the apnea-hypopnea index (AHI) served as the primary outcome. The percentage change in fitness (submaximal metabolic equivalents [METs]) and change in weight (kg) were the primary independent variables. Primary analyses collapsed intervention conditions with statistical adjustment for treatment group and baseline METs, weight, and AHI among other relevant covariates.
Results: At baseline, greater METs were associated with lower AHI (B [SE] = -1.48 [0.71], P = 0.038), but this relationship no longer existed (B [SE] = -0.24 [0.73], P = 0.75) after adjustment for weight (B [SE] = 0.31 [0.07], P < 0.0001). Fitness significantly increased at year 1 (+16.53 ± 28.71% relative to baseline), but returned to near-baseline levels by year 4 (+1.81 ± 24.48%). In mixed-model analyses of AHI change over time without consideration of weight change, increased fitness at year 1 (B [SE] = -0.15 [0.04], P < 0.0001), but not at year 4 (B [SE] = 0.04 [0.05], P = 0.48), was associated with AHI reduction. However, with weight change in the model, greater weight loss was associated with AHI reduction at years 1 and 4 (B [SE] = 0.81 [0.16] and 0.60 [0.16], both P < 0.0001), rendering the association between fitness and AHI change at year 1 nonsignificant (B [SE] = -0.04 [0.04], P = 0.31).
Conclusions: Among overweight/obese adults with type 2 diabetes, fitness change did not influence OSA severity change when weight change was taken into account.
Clinical trial registration: ClinicalTrials.gov identification number NCT00194259.
Keywords: apnea-hypopnea index; cardiorespiratory fitness; obstructive sleep apnea; physical activity; weight loss.
© 2016 Associated Professional Sleep Societies, LLC.
Figures
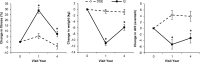
Figure 1
Mean changes (adjusted least-squares means ± standard error) in fitness, body weight, and apnea-hypopnea index (AHI) from baseline at years 1 and 4 according to intervention group. The dashed line (open circles) indicates the Diabetes Support and Education (DSE) group; the solid line (closed squares) indicates the Intensive Lifestyle Intervention (ILI) group. The asterisk indicates a significant difference between the DSE and ILI groups (P < 0.05).
Similar articles
- Sleep architecture following a weight loss intervention in overweight and obese patients with obstructive sleep apnea and type 2 diabetes: relationship to apnea-hypopnea index.
Shechter A, St-Onge MP, Kuna ST, Zammit G, RoyChoudhury A, Newman AB, Millman RP, Reboussin DM, Wadden TA, Jakicic JM, Pi-Sunyer FX, Wing RR, Foster GD; Sleep AHEAD Research Group of the Look AHEAD Research Group. Shechter A, et al. J Clin Sleep Med. 2014 Nov 15;10(11):1205-11. doi: 10.5664/jcsm.4202. J Clin Sleep Med. 2014. PMID: 25325608 Free PMC article. Clinical Trial. - Effects of a lifestyle intervention on REM sleep-related OSA severity in obese individuals with type 2 diabetes.
Shechter A, Foster GD, Lang W, Reboussin DM, St-Onge MP, Zammit G, Newman AB, Millman RP, Wadden TA, Jakicic JM, Strotmeyer ES, Wing RR, Pi-Sunyer FX, Kuna ST; Sleep Ahead Research Group of the Look Ahead Research Group. Shechter A, et al. J Sleep Res. 2017 Dec;26(6):747-755. doi: 10.1111/jsr.12559. Epub 2017 May 31. J Sleep Res. 2017. PMID: 28560832 Free PMC article. Clinical Trial. - A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study.
Foster GD, Borradaile KE, Sanders MH, Millman R, Zammit G, Newman AB, Wadden TA, Kelley D, Wing RR, Pi-Sunyer FX, Reboussin D, Kuna ST; Sleep AHEAD Research Group of Look AHEAD Research Group. Foster GD, et al. Arch Intern Med. 2009 Sep 28;169(17):1619-26. doi: 10.1001/archinternmed.2009.266. Arch Intern Med. 2009. PMID: 19786682 Free PMC article. Clinical Trial. - Bariatric Surgery or Non-Surgical Weight Loss for Obstructive Sleep Apnoea? A Systematic Review and Comparison of Meta-analyses.
Ashrafian H, Toma T, Rowland SP, Harling L, Tan A, Efthimiou E, Darzi A, Athanasiou T. Ashrafian H, et al. Obes Surg. 2015 Jul;25(7):1239-50. doi: 10.1007/s11695-014-1533-2. Obes Surg. 2015. PMID: 25537297 Review. - Surgical and Nonsurgical Weight Loss for Patients with Obstructive Sleep Apnea.
Saunders KH, Igel LI, Tchang BG. Saunders KH, et al. Otolaryngol Clin North Am. 2020 Jun;53(3):409-420. doi: 10.1016/j.otc.2020.02.003. Epub 2020 Apr 23. Otolaryngol Clin North Am. 2020. PMID: 32334866 Review.
Cited by
- The Influence of CPAP Therapy on Basal Metabolic Rate and Physical Activity in Obese Patients with Obstructive Sleep Apnea.
Siopi D, Steiropoulos P. Siopi D, et al. Nutrients. 2023 Oct 20;15(20):4446. doi: 10.3390/nu15204446. Nutrients. 2023. PMID: 37892521 Free PMC article. - American Association of Clinical Endocrinology Clinical Practice Guideline: Developing a Diabetes Mellitus Comprehensive Care Plan-2022 Update.
Blonde L, Umpierrez GE, Reddy SS, McGill JB, Berga SL, Bush M, Chandrasekaran S, DeFronzo RA, Einhorn D, Galindo RJ, Gardner TW, Garg R, Garvey WT, Hirsch IB, Hurley DL, Izuora K, Kosiborod M, Olson D, Patel SB, Pop-Busui R, Sadhu AR, Samson SL, Stec C, Tamborlane WV Jr, Tuttle KR, Twining C, Vella A, Vellanki P, Weber SL. Blonde L, et al. Endocr Pract. 2022 Oct;28(10):923-1049. doi: 10.1016/j.eprac.2022.08.002. Epub 2022 Aug 11. Endocr Pract. 2022. PMID: 35963508 Free PMC article. - Sleep Apnea in Type 2 Diabetes.
Doumit J, Prasad B. Doumit J, et al. Diabetes Spectr. 2016 Feb;29(1):14-9. doi: 10.2337/diaspect.29.1.14. Diabetes Spectr. 2016. PMID: 26912960 Free PMC article. - Sleep-disordered breathing, sleep apnea, and other obesity-related sleep disorders: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022.
Pennings N, Golden L, Yashi K, Tondt J, Bays HE. Pennings N, et al. Obes Pillars. 2022 Nov 15;4:100043. doi: 10.1016/j.obpill.2022.100043. eCollection 2022 Dec. Obes Pillars. 2022. PMID: 37990672 Free PMC article. - Use of polysomnography and home sleep apnea tests for the longitudinal management of obstructive sleep apnea in adults: an American Academy of Sleep Medicine clinical guidance statement.
Caples SM, Anderson WM, Calero K, Howell M, Hashmi SD. Caples SM, et al. J Clin Sleep Med. 2021 Jun 1;17(6):1287-1293. doi: 10.5664/jcsm.9240. J Clin Sleep Med. 2021. PMID: 33704050 Free PMC article. Review.
References
- Ferini-Strambi L, Baietto C, Di Gioia MR, et al. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): partial reversibility after continuous positive airway pressure (CPAP) Brain Res Bull. 2003;61:87–92. - PubMed
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoeahypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 DK057151/DK/NIDDK NIH HHS/United States
- K23 HL118318/HL/NHLBI NIH HHS/United States
- U01 DK057135/DK/NIDDK NIH HHS/United States
- U01 DK057219/DK/NIDDK NIH HHS/United States
- U01 DK057154/DK/NIDDK NIH HHS/United States
- DK057135/DK/NIDDK NIH HHS/United States
- U01 DK056992/DK/NIDDK NIH HHS/United States
- U01 DK057182/DK/NIDDK NIH HHS/United States
- DK60426/DK/NIDDK NIH HHS/United States
- U01 DK057136/DK/NIDDK NIH HHS/United States
- U01 DK057002/DK/NIDDK NIH HHS/United States
- U01 DK057177/DK/NIDDK NIH HHS/United States
- HL070301/HL/NHLBI NIH HHS/United States
- U01 DK057131/DK/NIDDK NIH HHS/United States
- HL118318/HL/NHLBI NIH HHS/United States
- P30 DK026687/DK/NIDDK NIH HHS/United States
- U01 DK057178/DK/NIDDK NIH HHS/United States
- DK56992/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials