Human Intestinal Enteroids: a New Model To Study Human Rotavirus Infection, Host Restriction, and Pathophysiology - PubMed (original) (raw)
. 2015 Oct 7;90(1):43-56.
doi: 10.1128/JVI.01930-15. Print 2016 Jan 1.
Sarah E Blutt 1, Khalil Ettayebi 1, Xi-Lei Zeng 1, James R Broughman 1, Sue E Crawford 1, Umesh C Karandikar 1, Narayan P Sastri 1, Margaret E Conner 1, Antone R Opekun 2, David Y Graham 3, Waqar Qureshi 2, Vadim Sherman 4, Jennifer Foulke-Abel 5, Julie In 5, Olga Kovbasnjuk 5, Nicholas C Zachos 5, Mark Donowitz 5, Mary K Estes 6
Affiliations
- PMID: 26446608
- PMCID: PMC4702582
- DOI: 10.1128/JVI.01930-15
Human Intestinal Enteroids: a New Model To Study Human Rotavirus Infection, Host Restriction, and Pathophysiology
Kapil Saxena et al. J Virol. 2015.
Abstract
Human gastrointestinal tract research is limited by the paucity of in vitro intestinal cell models that recapitulate the cellular diversity and complex functions of human physiology and disease pathology. Human intestinal enteroid (HIE) cultures contain multiple intestinal epithelial cell types that comprise the intestinal epithelium (enterocytes and goblet, enteroendocrine, and Paneth cells) and are physiologically active based on responses to agonists. We evaluated these nontransformed, three-dimensional HIE cultures as models for pathogenic infections in the small intestine by examining whether HIEs from different regions of the small intestine from different patients are susceptible to human rotavirus (HRV) infection. Little is known about HRVs, as they generally replicate poorly in transformed cell lines, and host range restriction prevents their replication in many animal models, whereas many animal rotaviruses (ARVs) exhibit a broader host range and replicate in mice. Using HRVs, including the Rotarix RV1 vaccine strain, and ARVs, we evaluated host susceptibility, virus production, and cellular responses of HIEs. HRVs infect at higher rates and grow to higher titers than do ARVs. HRVs infect differentiated enterocytes and enteroendocrine cells, and viroplasms and lipid droplets are induced. Heterogeneity in replication was seen in HIEs from different patients. HRV infection and RV enterotoxin treatment of HIEs caused physiological lumenal expansion detected by time-lapse microscopy, recapitulating one of the hallmarks of rotavirus-induced diarrhea. These results demonstrate that HIEs are a novel pathophysiological model that will allow the study of HRV biology, including host restriction, cell type restriction, and virus-induced fluid secretion.
Importance: Our research establishes HIEs as nontransformed cell culture models to understand human intestinal physiology and pathophysiology and the epithelial response, including host restriction of gastrointestinal infections such as HRV infection. HRVs remain a major worldwide cause of diarrhea-associated morbidity and mortality in children ≤5 years of age. Current in vitro models of rotavirus infection rely primarily on the use of animal rotaviruses because HRV growth is limited in most transformed cell lines and animal models. We demonstrate that HIEs are novel, cellularly diverse, and physiologically relevant epithelial cell cultures that recapitulate in vivo properties of HRV infection. HIEs will allow the study of HRV biology, including human host-pathogen and live, attenuated vaccine interactions; host and cell type restriction; virus-induced fluid secretion; cell-cell communication within the epithelium; and the epithelial response to infection in cultures from genetically diverse individuals. Finally, drug therapies to prevent/treat diarrheal disease can be tested in these physiologically active cultures.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
FIG 1
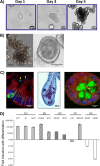
Characterization of differentiated human jejunal enteroids. (A) Representative images of jejunal enteroids grown over 5 days from intestinal crypts (bar = 50 μm). (B) After 5 days of growth in complete medium with growth factors (CMGF+ medium), enteroids typically result in two major morphologies, multilobular (left) (bar = 150 μm) and cystic (right) (bar = 100 μm). (C) Upon differentiation, enteroids contain the four major mature cell types of the small intestinal epithelium. (Left) Chromogranin A-containing enteroendocrine cells (green) and sucrase-isomaltase-expressing enterocytes (arrowheads) (bar = 10 μm). (Middle) Periodic acid-Schiff stain-reacting goblet cells (purple) (bar = 20 μm). (Right) Lysozyme-containing Paneth cells (green) (bar = 10 μm). E-cadherin (red) and DAPI (4′,6-diamidino-2-phenylindole) nuclear staining (blue) are shown in the left and right panels. (D) qRT-PCR results showing the fold change in levels of transcripts in differentiated enteroids relative to the transcript levels in undifferentiated enteroids. Transcript levels were first normalized to GAPDH levels prior to obtaining the relative fold change by using the 2−ΔΔ_CT_ method. Shown are markers for enterocytes (EC), enteroendocrine cells (EE), goblet cells (GC), Paneth cells (PC), and stem cells (SC). Gene symbols represent lactase (LCT), sucrase-isomaltase (SI), alkaline phosphatase (ALPI), chromogranin A (CHGA), synaptophysin (SYP), mucin 2 (MUC2), trefoil factor 3 (TFF3), lysozyme (LYZ), defensin alpha 5 (DEFA5), antigen identified by monoclonal antibody Ki-67 (MKI67), and leucine-rich-repeat-containing G-protein-coupled receptor 5 (LGR5) genes. Error bars indicate standard errors of the means (n = 3).
FIG 2
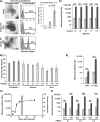
Human rotavirus infection and replication properties in human intestinal enteroids. (A) Jejunal enteroids from one patient (patient j11) were either mock infected or infected with RV at an MOI of 20 PFU/cell. At 20 hpi, enteroids were visualized by light microscopy (left) (bar = 50 μm) for cytopathic effect. Enteroids were also assessed for the percentage of infected cells by flow cytometry. Infected cells were defined as cells containing intracellular rotavirus antigen as detected by rabbit polyclonal antirotavirus serum. Examples of individual infection results (left) are accompanied by composite results from the experiment (right). (B) Enteroids generated from 11 different patients across the three sections of the small intestine were infected with HRV Ito at an MOI of 10 FFU/cell and assessed for the percentage of infected cells as described above for panel A. (C) A one-step growth curve for HRV Ito replication was performed over 30 h. Enteroids were infected at an MOI of 10 FFU/cell. At each of the 6 time points, enteroids and the surrounding supernatant were harvested, and the amount of infectious virus was quantified by a fluorescent-focus assay. Viral titer is displayed on the left y axis and is represented by black circles on the graph. Cytotoxicity was also measured at 2 hpi and 14 hpi and is represented as bars, with values displayed on the right y axis. (D) Jejunal enteroids from 5 different patients were infected with HRV Ito at an MOI of 0.5 FFU/cell, and the amount of infectious virus was quantified as described above for panel C at 2 hpi and 24 hpi. (E) Jejunal enteroids from one patient (patient j11) were infected with either RRV or HRV at an MOI of 0.5 FFU/cell, and the amount of infectious virus was quantified at 1.5 hpi and 24 hpi. (F) Jejunal enteroids from three patients were infected with either RV1 (Rotarix) or Wa, and the amount of infectious virus was quantified at 2 hpi and 24 hpi. In panels D to F, numbers above the bars show fold increases from 1.5 or 2 hpi (light gray bars) to 24 hpi (dark gray bars). (G) Secretor-negative enteroids from 3 different patients were infected with HRV strains Ito (left), Wa (middle), and RV1 (right) at an MOI of 0.5 FFU/cell. The amount of infectious virus at 1.5 hpi and 24 hpi was quantified as described above for panel C. Each bar represents the fold increase in viral growth from 1.5 hpi to 24 hpi. Statistical analysis was performed by using one-way ANOVA, followed by post hoc analysis with a Tukey HSD test. (H) Electron micrograph of an infected cell within an enteroid. Strain Ito particles (RV) adjacent to a lipid droplet (LD) and viroplasm (V) are shown (bar = 250 nm). Results are representative of data from duplicate (B, D, and F) or triplicate (A, C, and E) independent experiments. Each data bar represents means ± standard deviations for 3 samples within each independent experiment. Statistical analyses were performed by using Student's t test unless otherwise specified.
FIG 2
Human rotavirus infection and replication properties in human intestinal enteroids. (A) Jejunal enteroids from one patient (patient j11) were either mock infected or infected with RV at an MOI of 20 PFU/cell. At 20 hpi, enteroids were visualized by light microscopy (left) (bar = 50 μm) for cytopathic effect. Enteroids were also assessed for the percentage of infected cells by flow cytometry. Infected cells were defined as cells containing intracellular rotavirus antigen as detected by rabbit polyclonal antirotavirus serum. Examples of individual infection results (left) are accompanied by composite results from the experiment (right). (B) Enteroids generated from 11 different patients across the three sections of the small intestine were infected with HRV Ito at an MOI of 10 FFU/cell and assessed for the percentage of infected cells as described above for panel A. (C) A one-step growth curve for HRV Ito replication was performed over 30 h. Enteroids were infected at an MOI of 10 FFU/cell. At each of the 6 time points, enteroids and the surrounding supernatant were harvested, and the amount of infectious virus was quantified by a fluorescent-focus assay. Viral titer is displayed on the left y axis and is represented by black circles on the graph. Cytotoxicity was also measured at 2 hpi and 14 hpi and is represented as bars, with values displayed on the right y axis. (D) Jejunal enteroids from 5 different patients were infected with HRV Ito at an MOI of 0.5 FFU/cell, and the amount of infectious virus was quantified as described above for panel C at 2 hpi and 24 hpi. (E) Jejunal enteroids from one patient (patient j11) were infected with either RRV or HRV at an MOI of 0.5 FFU/cell, and the amount of infectious virus was quantified at 1.5 hpi and 24 hpi. (F) Jejunal enteroids from three patients were infected with either RV1 (Rotarix) or Wa, and the amount of infectious virus was quantified at 2 hpi and 24 hpi. In panels D to F, numbers above the bars show fold increases from 1.5 or 2 hpi (light gray bars) to 24 hpi (dark gray bars). (G) Secretor-negative enteroids from 3 different patients were infected with HRV strains Ito (left), Wa (middle), and RV1 (right) at an MOI of 0.5 FFU/cell. The amount of infectious virus at 1.5 hpi and 24 hpi was quantified as described above for panel C. Each bar represents the fold increase in viral growth from 1.5 hpi to 24 hpi. Statistical analysis was performed by using one-way ANOVA, followed by post hoc analysis with a Tukey HSD test. (H) Electron micrograph of an infected cell within an enteroid. Strain Ito particles (RV) adjacent to a lipid droplet (LD) and viroplasm (V) are shown (bar = 250 nm). Results are representative of data from duplicate (B, D, and F) or triplicate (A, C, and E) independent experiments. Each data bar represents means ± standard deviations for 3 samples within each independent experiment. Statistical analyses were performed by using Student's t test unless otherwise specified.
FIG 3
Effect of differentiation status on susceptibility to HRV infection. (A) Undifferentiated and differentiated jejunal enteroids from the same patient were either mock infected or infected with HRV Ito at an MOI of 10 FFU/cell. At 20 hpi, single-cell suspensions were assessed for the presence of intracellular rotavirus antigen by flow cytometry. Results are representative of data from triplicate independent experiments. Each data bar represents the mean ± standard deviation for 3 samples within each independent experiment. (B) Undifferentiated and differentiated jejunal enteroids from the same patient (patient j2) were infected with HRV Ito at an MOI of 0.5 FFU/cell, and the amount of infectious virus was quantified at 1.5 hpi (light gray bars) and 24 hpi (dark gray bars). Fold increases are displayed above the bars. Results are representative of data from replicate experiments performed with enteroids from two additional patients (patients j3 and j11). Each data bar represents the mean ± standard deviation for 4 samples within each independent experiment. Statistical analyses were performed by using Student's t test.
FIG 4
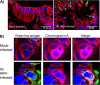
Assessment of differentiated cell types infected by HRV. Ileal enteroids were either mock infected or infected with HRV Ito at an MOI of 10, fixed at 10 hpi, processed for immunofluorescence staining, and visualized by using confocal microscopy. (A) Paraffin-embedded sections of mock-infected (left) and HRV-infected (right) ileal enteroids were assessed for intracellular rotavirus antigen (green), E-cadherin (red), and nuclei (blue). Infected enterocytes (arrows) are identified as E-cadherin-expressing cells containing rotavirus antigen (bar = 20 μm). (B) Enteroendocrine cell infection was assessed by visualizing rotavirus antigen-containing cells (green) (left), chromogranin A-containing cells (magenta) (middle), and the merged image (right). Staining of nuclei (blue) and E-cadherin (red) is present in all panels (bar = 10 μm).
FIG 5
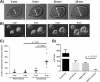
Fluid dynamics of human intestinal enteroids in response to rotavirus. (A) Duodenal HIEs were treated with forskolin and imaged over 40 min. The white line marks the enteroid lumen diameter (bar = 50 μm). (B) Duodenal HIEs were infected with HRV Ito at an MOI of 20 PFU/cell. HIEs were imaged from 2 hpi to 12 hpi. Images from four representative time points are shown. White lines demarcate the lumen diameter (bar = 50 μm). (C) Duodenal HIEs were either mock infected or infected with HRV as described above for panel B. The lumenal radius and the total enteroid radius were measured at 2 hpi and 6 hpi for mock-infected (n = 10) and HRV-infected (n = 9) HIEs. The ratio of these two radii is shown as lumen radius/total enteroid radius at 2 hpi and at 6 hpi. Black bars represent median values. (D) Duodenal HIEs were treated with forskolin (20 μM), carbachol (200 μM), the NSP4 wild-type (WT) peptide spanning residues 95 to 146 (20 μM), or the E120A/Q123A mutant NSP4 peptide spanning residues 95 to 146 (20 μM). Forskolin-treated HIEs (n = 5) were imaged for 10 min. Carbachol (n = 7)-, NSP4 wild-type peptide (n = 10)-, and NSP4 mutant peptide (n = 5)-treated HIEs were imaged for 40 min, and the rate of increase in the cross-sectional area is shown in square micrometers per minute. Statistical analyses of data in panels C and D were performed by using Student's t test.
Similar articles
- Human Intestinal Enteroids: New Models to Study Gastrointestinal Virus Infections.
Zou WY, Blutt SE, Crawford SE, Ettayebi K, Zeng XL, Saxena K, Ramani S, Karandikar UC, Zachos NC, Estes MK. Zou WY, et al. Methods Mol Biol. 2019;1576:229-247. doi: 10.1007/7651_2017_1. Methods Mol Biol. 2019. PMID: 28361480 Free PMC article. - Human Sapovirus Replication in Human Intestinal Enteroids.
Euller-Nicolas G, Le Mennec C, Schaeffer J, Zeng XL, Ettayebi K, Atmar RL, Le Guyader FS, Estes MK, Desdouits M. Euller-Nicolas G, et al. J Virol. 2023 Apr 27;97(4):e0038323. doi: 10.1128/jvi.00383-23. Epub 2023 Apr 11. J Virol. 2023. PMID: 37039654 Free PMC article. - Infection of porcine small intestinal enteroids with human and pig rotavirus A strains reveals contrasting roles for histo-blood group antigens and terminal sialic acids.
Guo Y, Candelero-Rueda RA, Saif LJ, Vlasova AN. Guo Y, et al. PLoS Pathog. 2021 Jan 29;17(1):e1009237. doi: 10.1371/journal.ppat.1009237. eCollection 2021 Jan. PLoS Pathog. 2021. PMID: 33513201 Free PMC article. - A viral enterotoxin. A new mechanism of virus-induced pathogenesis.
Estes MK, Morris AP. Estes MK, et al. Adv Exp Med Biol. 1999;473:73-82. Adv Exp Med Biol. 1999. PMID: 10659345 Review. - Lipid droplets form complexes with viroplasms and are crucial for rotavirus replication.
Crawford SE, Desselberger U. Crawford SE, et al. Curr Opin Virol. 2016 Aug;19:11-5. doi: 10.1016/j.coviro.2016.05.008. Epub 2016 Jun 21. Curr Opin Virol. 2016. PMID: 27341619 Free PMC article. Review.
Cited by
- An Optimized Reverse Genetics System Suitable for Efficient Recovery of Simian, Human, and Murine-Like Rotaviruses.
Sánchez-Tacuba L, Feng N, Meade NJ, Mellits KH, Jaïs PH, Yasukawa LL, Resch TK, Jiang B, López S, Ding S, Greenberg HB. Sánchez-Tacuba L, et al. J Virol. 2020 Aug 31;94(18):e01294-20. doi: 10.1128/JVI.01294-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32759316 Free PMC article. - Human mini-guts: new insights into intestinal physiology and host-pathogen interactions.
In JG, Foulke-Abel J, Estes MK, Zachos NC, Kovbasnjuk O, Donowitz M. In JG, et al. Nat Rev Gastroenterol Hepatol. 2016 Nov;13(11):633-642. doi: 10.1038/nrgastro.2016.142. Epub 2016 Sep 28. Nat Rev Gastroenterol Hepatol. 2016. PMID: 27677718 Free PMC article. Review. - Gastrointestinal organoids in the study of viral infections.
Gebert JT, Scribano F, Engevik KA, Perry JL, Hyser JM. Gebert JT, et al. Am J Physiol Gastrointest Liver Physiol. 2023 Jan 1;324(1):G51-G59. doi: 10.1152/ajpgi.00152.2022. Epub 2022 Nov 22. Am J Physiol Gastrointest Liver Physiol. 2023. PMID: 36414538 Free PMC article. Review. - Drug Discovery via Human-Derived Stem Cell Organoids.
Liu F, Huang J, Ning B, Liu Z, Chen S, Zhao W. Liu F, et al. Front Pharmacol. 2016 Sep 22;7:334. doi: 10.3389/fphar.2016.00334. eCollection 2016. Front Pharmacol. 2016. PMID: 27713700 Free PMC article. Review. - The Significance of the Intestinal Microbiome for Vaccinology: From Correlations to Therapeutic Applications.
Harris VC. Harris VC. Drugs. 2018 Jul;78(11):1063-1072. doi: 10.1007/s40265-018-0941-3. Drugs. 2018. PMID: 29943376 Free PMC article. Review.
References
- Foulke-Abel J, In J, Kovbasnjuk O, Zachos NC, Ettayebi K, Blutt SE, Hyser JM, Zeng XL, Crawford SE, Broughman JR, Estes MK, Donowitz M. 2014. Human enteroids as an ex-vivo model of host-pathogen interactions in the gastrointestinal tract. Exp Biol Med (Maywood) 239:1124–1134. doi:10.1177/1535370214529398. - DOI - PMC - PubMed
- Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. 2011. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141:1762–1772. doi:10.1053/j.gastro.2011.07.050. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
- R24 DK099803/DK/NIDDK NIH HHS/United States
- 570076890/Howard Hughes Medical Institute/United States
- P30 DK089502/DK/NIDDK NIH HHS/United States
- R01 AI080656/AI/NIAID NIH HHS/United States
- U19-AI116497/AI/NIAID NIH HHS/United States
- U19 AI116497/AI/NIAID NIH HHS/United States
- R01 DK026523/DK/NIDDK NIH HHS/United States
- U54 HD007495/HD/NICHD NIH HHS/United States
- P30 CA125123/CA/NCI NIH HHS/United States
- P30 DK056338/DK/NIDDK NIH HHS/United States
- T32 DK007632/DK/NIDDK NIH HHS/United States
- U18 TR000552/TR/NCATS NIH HHS/United States
- U18-TR000552/TR/NCATS NIH HHS/United States
- P01 AI057788/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical