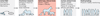Companion animals: Translational scientist's new best friends - PubMed (original) (raw)
Review
Companion animals: Translational scientist's new best friends
Amir Kol et al. Sci Transl Med. 2015.
Abstract
Knowledge and resources derived from veterinary medicine represent an underused resource that could serve as a bridge between data obtained from diseases models in laboratory animals and human clinical trials. Naturally occurring disease in companion animals that display the defining attributes of similar, if not identical, diseases in humans hold promise for providing predictive proof of concept in the evaluation of new therapeutics and devices. Here we outline comparative aspects of naturally occurring diseases in companion animals and discuss their current uses in translational medicine, benefits, and shortcomings. Last, we envision how these natural models of disease might ultimately decrease the failure rate in human clinical trials and accelerate the delivery of effective treatments to the human clinical market.
Copyright © 2015, American Association for the Advancement of Science.
Figures
Fig. 1. Companion animal trials
Shown (highlighted in red) is the proposed role of veterinary clinical trials as predictors of human efficacy studies. Veterinary clinical trials could serve as a bridge between preclinical and clinical studies, with the goal of reducing the failure rates of human clinical trials and accelerating approval of new therapeutics. R&D, research and development.
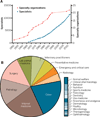
Fig. 2. Clinical specialization in veterinary medicine
The numbers of board-certified veterinary specialists and specialty organizations since 1950 (A) and the current distribution of veterinary specialists (B) are depicted. In 1951, the American College of Veterinary Pathologists was recognized as the first specialty organization. Since then, the American Veterinary Medical Association’s, American Board of Veterinary Specialties has given accreditation to 21 additional specialty organizations. The most recent addition, the American College of Animal Welfare, was accredited in 2012. Congruous with the expansion of recognized specialties, the numbers of board-certified specialists is also on the rise. A small community of 389 veterinary specialists in the year 1967 has increased exponentially throughout the intervening years. At the time of this writing, there are more than 11,000 active board-certified veterinary specialists.
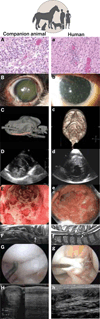
Fig. 3. Behaving like animals
Here, we show similar clinical findings in veterinary and human patients with related naturally occurring diseases. Histologic photomicrographs (H&E) from a dog (A) and a human (a) with glioblastoma depict similar morphological features, including vascular proliferation. Close-up images of a dog (B) and a human (b) eye with KCS depict ocular surface changes. A computed tomographic three-dimensional reconstruction image of a dog (C) that received mandibular reconstruction using rh-BMP2 infused in a scaffold and a human (c) who has undergone mandibular reconstruction using autologous bone grafting. An echocardiogram from a cat (D) and a human (d) with hypertrophic cardiomyopathy demonstrates concentric hypertrophy of the left ventricle characterized by thickening of the left ventricular walls with decreased ventricular volume. A colonoscopic image from a dog (E) and a human (e) with inflammatory bowel disease demonstrates colonic inflammation, hemorrhage, and ulceration. A contrast-enhanced magnetic resonance myelogram in a dog (F) and a human (f) with cervical spondylomyelopathy depicts vertebral canal stenosis (white arrows). An arthroscopic image of a torn cranial cruciate ligament in a dog (G) and a torn anterior cruciate ligament in a knee of a human (g) reveals associated arthritic changes. Ultrasonographic images of a superficial digital flexor tendon lesion in a horse (H) and Achilles tendonitis in a human (h) demonstrate similar core lesions.
Similar articles
- Cell Therapy in Veterinary Medicine as a Proof-of-Concept for Human Therapies: Perspectives From the North American Veterinary Regenerative Medicine Association.
Arzi B, Webb TL, Koch TG, Volk SW, Betts DH, Watts A, Goodrich L, Kallos MS, Kol A. Arzi B, et al. Front Vet Sci. 2021 Nov 30;8:779109. doi: 10.3389/fvets.2021.779109. eCollection 2021. Front Vet Sci. 2021. PMID: 34917671 Free PMC article. - An eye on the dog as the scientist's best friend for translational research in ophthalmology: Focus on the ocular surface.
Sebbag L, Mochel JP. Sebbag L, et al. Med Res Rev. 2020 Nov;40(6):2566-2604. doi: 10.1002/med.21716. Epub 2020 Jul 31. Med Res Rev. 2020. PMID: 32735080 Review. - Craniomaxillofacial Disorders and Solutions in Humans and Animals.
Arzi B, Moshaverinia A, Verstraete FJM, Fiani N, Nishimura I. Arzi B, et al. J Dent Res. 2018 Apr;97(4):364-370. doi: 10.1177/0022034518758017. Epub 2018 Feb 26. J Dent Res. 2018. PMID: 29481293 Free PMC article. - Companion animal models of neurological disease.
Partridge B, Rossmeisl JH Jr. Partridge B, et al. J Neurosci Methods. 2020 Feb 1;331:108484. doi: 10.1016/j.jneumeth.2019.108484. Epub 2019 Nov 13. J Neurosci Methods. 2020. PMID: 31733285 Free PMC article. Review. - Concise Review: Stem Cell Trials Using Companion Animal Disease Models.
Hoffman AM, Dow SW. Hoffman AM, et al. Stem Cells. 2016 Jul;34(7):1709-29. doi: 10.1002/stem.2377. Epub 2016 May 3. Stem Cells. 2016. PMID: 27066769 Review.
Cited by
- 3D-printed nerve guidance conduits multi-functionalized with canine multipotent mesenchymal stromal cells promote neuroregeneration after sciatic nerve injury in rats.
Rodríguez-Sánchez DN, Pinto GBA, Cartarozzi LP, de Oliveira ALR, Bovolato ALC, de Carvalho M, da Silva JVL, Dernowsek JA, Golim M, Barraviera B, Ferreira RS, Deffune E, Bertanha M, Amorim RM. Rodríguez-Sánchez DN, et al. Stem Cell Res Ther. 2021 May 29;12(1):303. doi: 10.1186/s13287-021-02315-8. Stem Cell Res Ther. 2021. PMID: 34051869 Free PMC article. - Dogs are man's best friend: in sickness and in health.
Bentley RT, Ahmed AU, Yanke AB, Cohen-Gadol AA, Dey M. Bentley RT, et al. Neuro Oncol. 2017 Mar 1;19(3):312-322. doi: 10.1093/neuonc/now109. Neuro Oncol. 2017. PMID: 27298310 Free PMC article. Review. - 3D printing for surgical planning of canine oral and maxillofacial surgeries.
Huang YH, Lee B, Chuy JA, Goldschmidt SL. Huang YH, et al. 3D Print Med. 2022 Jun 9;8(1):17. doi: 10.1186/s41205-022-00142-y. 3D Print Med. 2022. PMID: 35678954 Free PMC article. - Navigating regulatory pathways for translation of biologic cartilage repair products.
Nordberg RC, Otarola GA, Wang D, Hu JC, Athanasiou KA. Nordberg RC, et al. Sci Transl Med. 2022 Aug 24;14(659):eabp8163. doi: 10.1126/scitranslmed.abp8163. Epub 2022 Aug 24. Sci Transl Med. 2022. PMID: 36001677 Free PMC article. Review. - Concise Review: Canine Diabetes Mellitus as a Translational Model for Innovative Regenerative Medicine Approaches.
Moshref M, Tangey B, Gilor C, Papas KK, Williamson P, Loomba-Albrecht L, Sheehy P, Kol A. Moshref M, et al. Stem Cells Transl Med. 2019 May;8(5):450-455. doi: 10.1002/sctm.18-0163. Epub 2019 Feb 5. Stem Cells Transl Med. 2019. PMID: 30719867 Free PMC article. Review.
References
- Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J. Clinical development success rates for investigational drugs. Nat. Biotechnol. 2014;32:40–51. - PubMed
- Code of Federal Regulations - Title 21 - Food and Drugs. 2015 312.23 (a)(8) www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=312.23.
- Guidance for industry: Preclinical assessment of investigational cellular and gene therapy products. Washington, DC: FDA, Center for Biologics Evaluation and Research; 2013.
Publication types
MeSH terms
Grants and funding
- R01 CA123227/CA/NCI NIH HHS/United States
- P30 CA093373/CA/NCI NIH HHS/United States
- R01 CA081237/CA/NCI NIH HHS/United States
- R01 EY016134/EY/NEI NIH HHS/United States
- R01 EY019970/EY/NEI NIH HHS/United States
- R01 HL115205/HL/NHLBI NIH HHS/United States
- R21 DE024711/DE/NIDCR NIH HHS/United States
- 1R21DE024711-01/DE/NIDCR NIH HHS/United States
- R01 CA076069/CA/NCI NIH HHS/United States
- K01 OD011111/OD/NIH HHS/United States
- R01 CA102188/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources