Dynamic Regulation of N-Methyl-d-aspartate (NMDA) and α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) Receptors by Posttranslational Modifications - PubMed (original) (raw)
Review
Dynamic Regulation of N-Methyl-d-aspartate (NMDA) and α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) Receptors by Posttranslational Modifications
Marc P Lussier et al. J Biol Chem. 2015.
Abstract
Many molecular mechanisms underlie the changes in synaptic glutamate receptor content that are required by neuronal networks to generate cellular correlates of learning and memory. During the last decade, posttranslational modifications have emerged as critical regulators of synaptic transmission and plasticity. Notably, phosphorylation, ubiquitination, and palmitoylation control the stability, trafficking, and synaptic expression of glutamate receptors in the central nervous system. In the current review, we will summarize some of the progress made by the neuroscience community regarding our understanding of phosphorylation, ubiquitination, and palmitoylation of the NMDA and AMPA subtypes of glutamate receptors.
Keywords: N-methyl-D-aspartate receptor (NMDA receptor, NMDAR); alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA receptor, AMPAR); glutamate receptor; post-translational modification (PTM); protein palmitoylation; ubiquitin.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
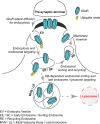
FIGURE 1.
Cellular mechanisms regulating synaptic expression of GluRs. The synaptic molecular content of iGluRs is controlled by multiple cellular events.
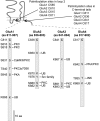
FIGURE 2.
PTMs decorate GluN2A and GluN2B C-terminal tails. The GluN2A and GluN2B C termini contain several amino acids (aa) modified by phosphorylation (serine (S) or tyrosine (Y)), ubiquitination (lysine K)), and palmitoylation (cysteine (C)). Kinases targeting a specific residue are illustrated.
FIGURE 3.
PTMs modify AMPAR intracellular domains. The AMPAR C termini are substrates for several kinases targeting serine (S), threonine (T), or tyrosine (Y). Also, AMPARs are modified by palmitoylation on cysteines (C) and ubiquitination (UB) on lysines (K). The amino acids (aa) targeted by specific PTMs are depicted.
FIGURE 4.
NMDAR lateral diffusion and endocytosis. GluN2B/2B receptor removal from synapses is controlled by the coordinated work of several kinases, including CaMKII, CK2, and Fyn/Src. In addition, PKC and Cdk5 may be involved in the process. The synaptic activity-dependent activation of CaMKII promotes phosphorylation on the PDZ ligand of GluN2B by CK2. This phosphorylation disrupts the interaction of the receptor with scaffolding proteins and leads to GluN2B internalization via dephosphorylation of the YEKL endocytic motif. See text for details.
Similar articles
- Palmitoylation-mediated synaptic regulation of AMPA receptor trafficking and function.
Sohn H, Park M. Sohn H, et al. Arch Pharm Res. 2019 May;42(5):426-435. doi: 10.1007/s12272-019-01134-z. Epub 2019 Mar 5. Arch Pharm Res. 2019. PMID: 30838509 Free PMC article. Review. - Posttranslational modifications and receptor-associated proteins in AMPA receptor trafficking and synaptic plasticity.
Jiang J, Suppiramaniam V, Wooten MW. Jiang J, et al. Neurosignals. 2006-2007;15(5):266-82. doi: 10.1159/000105517. Epub 2007 Jul 9. Neurosignals. 2006. PMID: 17622793 Review. - Neurokinin release in the rat nucleus of the solitary tract via NMDA and AMPA receptors.
Colin I, Blondeau C, Baude A. Colin I, et al. Neuroscience. 2002;115(4):1023-33. doi: 10.1016/s0306-4522(02)00541-9. Neuroscience. 2002. PMID: 12453476
Cited by
- Ouabain Modulates the Functional Interaction Between Na,K-ATPase and NMDA Receptor.
Akkuratov EE, Westin L, Vazquez-Juarez E, de Marothy M, Melnikova AK, Blom H, Lindskog M, Brismar H, Aperia A. Akkuratov EE, et al. Mol Neurobiol. 2020 Oct;57(10):4018-4030. doi: 10.1007/s12035-020-01984-5. Epub 2020 Jul 10. Mol Neurobiol. 2020. PMID: 32651756 Free PMC article. - Synaptic rearrangement of NMDA receptors controls memory engram formation and malleability in the cortex.
Bessières B, Dupuis J, Groc L, Bontempi B, Nicole O. Bessières B, et al. Sci Adv. 2024 Aug 30;10(35):eado1148. doi: 10.1126/sciadv.ado1148. Epub 2024 Aug 30. Sci Adv. 2024. PMID: 39213354 Free PMC article. - Ion flux-independent NMDA receptor signaling.
Park DK, Stein IS, Zito K. Park DK, et al. Neuropharmacology. 2022 Jun 1;210:109019. doi: 10.1016/j.neuropharm.2022.109019. Epub 2022 Mar 9. Neuropharmacology. 2022. PMID: 35278420 Free PMC article. Review. - The NMDA receptor intracellular C-terminal domains reciprocally interact with allosteric modulators.
Sapkota K, Dore K, Tang K, Irvine M, Fang G, Burnell ES, Malinow R, Jane DE, Monaghan DT. Sapkota K, et al. Biochem Pharmacol. 2019 Jan;159:140-153. doi: 10.1016/j.bcp.2018.11.018. Epub 2018 Nov 29. Biochem Pharmacol. 2019. PMID: 30503374 Free PMC article. - Limb-Clasping Response in NMDA Receptor Palmitoylation-Deficient Mice.
Suzuki N, Oota-Ishigaki A, Kaizuka T, Itoh M, Yamazaki M, Natsume R, Abe M, Sakimura K, Mishina M, Hayashi T. Suzuki N, et al. Mol Neurobiol. 2024 Nov;61(11):9125-9135. doi: 10.1007/s12035-024-04166-9. Epub 2024 Apr 9. Mol Neurobiol. 2024. PMID: 38592586 Free PMC article.
References
- Bredt D. S., and Nicoll R. A. (2003) AMPA receptor trafficking at excitatory synapses. Neuron 40, 361–379 - PubMed
- Malenka R. C., and Bear M. F. (2004) LTP and LTD: an embarrassment of riches. Neuron 44, 5–21 - PubMed
- Malinow R., and Malenka R. C. (2002) AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 25, 103–126 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases