Mitochondrial division inhibitor 1 protects against mutant huntingtin-induced abnormal mitochondrial dynamics and neuronal damage in Huntington's disease - PubMed (original) (raw)
. 2015 Dec 20;24(25):7308-25.
doi: 10.1093/hmg/ddv429. Epub 2015 Oct 12.
Affiliations
- PMID: 26464486
- PMCID: PMC4664169
- DOI: 10.1093/hmg/ddv429
Mitochondrial division inhibitor 1 protects against mutant huntingtin-induced abnormal mitochondrial dynamics and neuronal damage in Huntington's disease
Maria Manczak et al. Hum Mol Genet. 2015.
Abstract
The objective of this study was to determine the protective effects of the mitochondrial division inhibitor 1 (Mdivi1) in striatal neurons that stably express mutant Htt (STHDhQ111/Q111) and wild-type (WT) Htt (STHDhQ7/Q7). Using gene expression analysis, biochemical methods, transmission electron microscopy (TEM) and confocal microscopy methods, we studied (i) mitochondrial and synaptic activities by measuring mRNA and the protein levels of mitochondrial and synaptic genes, (ii) mitochondrial function and (iii) ultra-structural changes in mutant Htt neurons relative to WT Htt neurons. We also studied these parameters in Mdivil-treated and untreated WT and mutant Htt neurons. Increased expressions of mitochondrial fission genes, decreased expression of fusion genes and synaptic genes were found in the mutant Htt neurons relative to the WT Htt neurons. Electron microscopy of the mutant Htt neurons revealed a significantly increased number of mitochondria, indicating that mutant Htt fragments mitochondria. Biochemical analysis revealed defective mitochondrial functioning. In the Mdivil-treated mutant Htt neurons, fission genes were down-regulated, and fusion genes were up-regulated, suggesting that Mdivil decreases fission activity. Synaptic genes were up-regulated, and mitochondrial function was normal in the Mdivi1-treated mutant Htt neurons. Immunoblotting findings of mitochondrial and synaptic proteins agreed with mRNA findings. The TEM studies revealed that increased numbers of structurally intact mitochondria were present in Mdivi1-treated mutant Htt neurons. Increased synaptic and mitochondrial fusion genes and decreased fission genes were found in the Mdivi1-treated WT Htt neurons, indicating that Mdivi1 beneficially affects healthy neurons. Taken together, these findings suggest that Mdivi1 is protective against mutant Htt-induced mitochondrial and synaptic damage in HD neurons and that Mdivi1 may be a promising molecule for the treatment of HD patients.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
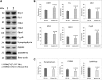
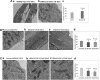
Figure 1.
Immunoblotting analysis of proteins in WT Htt (HDhQ7/Q7) and mutant Htt (HDhQ111/Q111) neurons. (A) Representative immunoblotting analysis of WT and mutant Htt neurons. (B) Quantitative densitometry analysis of mitochondrial dynamics and matrix proteins, Drp1, Fis1, Mfn1, Mfn2, Opa1 and CypD of WT Htt neurons relative to mutant Htt neurons. (C) Quantitative densitometry analysis of synaptic proteins synaptophysin, PSD95 and DARPP32 of mutant Htt neurons relative to WT Htt neurons. Significantly increased levels of fission proteins (Drp1 and Fis1) and significantly decreased levels of fusion and matrix proteins (Mfn1, Mfn2, Opa1 and CypD) in mutant Htt neurons relative to WT Htt neurons. Expression levels of synaptic protein levels synaptophysin and PSD95 and medium-spiny neuronal marker DARPP32 were significantly reduced in mutant Htt neurons.
Figure 2.
Immunoblotting analysis of proteins in WT Htt and mutant Htt neurons treated with Mdivi1 and untreated. (A) Representative immunoblotting analysis of WT and mutant Htt neurons. (B) Quantitative densitometry analysis of mitochondrial dynamics and matrix proteins, Drp1, Fis1, Mfn1, Mfn2, Opa1 and CypD of WT Htt neurons treated with Mdivi1 relative to Mdivi1 untreated. (C) Quantitative densitometry analysis of synaptic proteins synaptophysin, PSD and DARPP32 of WT Htt neurons treated with Mdivi1 relative to untreated. (D) Quantitative immunoblotting analysis of mitochondrial dynamics and matrix proteins Drp1, Fis1, Mfn1, Mfn2 and Opa1 and CypD proteins of mutant Htt neurons treated with Mdivi1 relative to untreated. (E) Quantitative densitometry analysis of synaptic proteins, synaptophysin, PSD and DARPP32 of mutant Htt neurons treated with Mdivi1 relative to untreated. The fission proteins Drp1 and Fis1 were significantly decreased, and the fusion proteins, Mfn1, Mfn2 and Opa1 and the synaptic, synaptophysin and PSD95 and DARPP32 proteins were significantly increased in Mdivil at 25 and 50 μ
m
concentrations, indicating that Mdivi1 reduces fission activity and enhances fusion and synaptic activity.
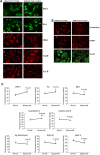
Figure 3.
Immunofluorescence analysis of proteins from WT Htt neurons and mutant Htt neurons. (A) Representative images of WT Htt and mutant Htt neurons from mitochondrial dynamics and matrix proteins. (B) Representative images of WT Htt and mutant Htt neurons from synaptic proteins. (C) Quantitative immunofluorescence analysis of mitochondrial dynamics and matrix and synaptic proteins, Drp1, Fis1, Mfn1, Mfn2, Opa1, CypD and synaptophysin, PSD95 and MAP2 of WT Htt and mutant Htt neurons.
Figure 4.
Immunofluorescence analysis of proteins from Mdivil-treated and untreated WT Htt neurons. (A) Representative images of Mdivi1-treated and untreated WT Htt neurons from mitochondrial dynamics and matrix proteins. (B) Representative images of Mdivi1-treated and untreated WT Htt neurons from synaptic proteins. (C) Quantitative immunofluorescence analysis of mitochondrial dynamics and matrix and synaptic proteins, Drp1, Fis1, Mfn1, Mfn2, Opa1, CypD and synaptophysin, PSD95 and MAP2 of WT Htt neurons treated with Mdivi1 and untreated. In the WT Htt neurons, the levels of both fission and the matrix proteins were significantly decreased, and the fusion protein and the ETC protein were significantly increased when treated with Mdivil at both 25 and 50 μ
m
concentrations.
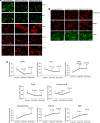
Figure 5.
Immunofluorescence analysis of proteins in Mdivil-treated and untreated mutant Htt neurons. (A) Representative images of Mdivi1-treated and untreated mutant Htt neurons from mitochondrial dynamics and matrix proteins. (B) Representative images of Mdivi1-treated and untreated mutant Htt neurons from synaptic proteins. (C) Quantitative immunofluorescence analysis of mitochondrial dynamics and matrix and synaptic proteins, Drp1, Fis1, Mfn1, Mfn2, Opa1, CypD and synaptophysin, PSD95 and MAP2 of mutant Htt (HDhQ111/Q111) neurons treated with Mdivi1 and untreated. The fission and matrix proteins were significantly decreased, and the fusion and ETC proteins were significantly increased upon treatment with Mdivil at 25 and 50 μ
m
concentrations, indicating that Mdivi1 reduces mitochondrial fission activity and enhances fusion activity.
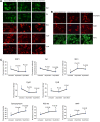
Figure 6.
Transmission electron microscopy of studies. (A) Mitochondrial number between WT Htt and mutant Htt neurons. (a) Healthy, intact mitochondria in the WT Htt neurons; (b) fragmented mitochondria in mutant Htt neurons. (c) Results from quantitative analysis of mitochondria. Significantly increased numbers of mitochondria were found in the mutant Htt neurons (P = 0.04) relative to WT Htt neurons. (B) Transmission electron microscopy of Mdivil-treated and untreated WT Htt neurons. (a) Healthy, intact mitochondria in the WT Htt neurons; (b) healthy, intact and elongated mitochondria in the 25 μ
m
Mdivi1-treated WT Htt neurons. (c) Healthy, intact mitochondria in the 50 μ
m
Mdivi1-treated WT Htt neurons. (d) Results from quantitative analysis of mitochondria. Significantly decreased numbers of mitochondria were found in the WT neurons treated with Mdivil at 25 μ
m
(P = 0.04) and 50 μ
m
(P = 0.04) concentrations compared with the mitochondria in mutant Htt neurons untreated with Mdivi1. (C) Transmission electron microscopy of Mdivil-treated and untreated mutant Htt neurons. (a) Fragmented and structurally damaged mitochondria in the mutant Htt neurons; (b) intact mitochondria in the 25 μ
m
Mdivi1-treated WT Htt neurons. (c) Intact mitochondria in the 50 μ
m
Mdivi1-treated mutant Htt neurons. (d) Results from quantitative analysis of mitochondria. Significantly decreased numbers of mitochondria were found in the mutant neurons treated with Mdivil at 25 μ
m
(P = 0.03) and 50 μ
m
(P = 0.02) concentrations compared with the mitochondria in mutant Htt neurons untreated with Mdivi1.
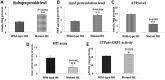
Figure 7.
Mitochondrial functional parameters in WT Htt and mutant Htt neurons. Mitochondrial function was assessed in WT Htt (HDhQ7/HDhQ7) and mutant Htt (HDhQ111/Q111) neurons by measuring: (A) H2O2 production, (B) lipid peroxidation, (C) ATP levels**,** (D) cell viability and (E) GTPase Drp1 activity. Significantly increased levels of H2O2 (P = 0.001) and 4-hydroxy-2-nonenol (P = 0.004), and significantly decreased ATP production (P = 0.001) and cell viability (P = 0.002) were found in the mutant Htt neurons relative to the WT Htt neurons. The level of GTPase Drp1 activity was significantly increased (P = 0.04) in mutant Htt neurons.
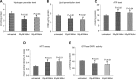
Figure 8.
Mitochondrial functional parameters in Mdivil-treated and untreated WT Htt neurons. Mitochondrial function was assessed in WT Htt neurons by measuring: (A) H2O2 production, (B) lipid peroxidation, (C) ATP levels, (D) cell viability and (E) GTPase Drp1 activity. Significantly decreased levels of the following mitochondrial functional parameters were found in the WT Htt neurons upon treatment with Mdivil: H2O2 with Mdivil at 25 μ
m
(P = 0.02) and 50 μ
m
(P = 0.04) concentrations; 4-hydroxy-2-nonenol with Mdivil at 25 μ
m
(P = 0.01) and 50 μ
m
(P = 0.02) concentrations; and GTPase Drp1 activity with Mdivil at 25 μ
m
(P = 0.01) and 50 μ
m
(P = 0.01) concentrations. In contrast, significantly increased mitochondrial functional parameters were found in the WT Htt neurons upon treatment with Mdivil: ATP at 25 μ
m
(P = 0.02) and 50 μ
m
(P = 0.04) concentrations, and cell viability at 25 μ
m
(P = 0.01) and 50 μ
m
(P = 0.01) concentrations.
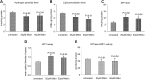
Figure 9.
Mitochondrial functional parameters in Mdivil-treated and untreated mutant Htt neurons. Mitochondrial function was assessed by measuring: (A) H2O2 production, (B) lipid peroxidation, (C) ATP levels, (D) cell viability and (E) GTPase Drp1 activity. Significantly decreased levels were found in the following parameters in mutant Htt neurons upon Mdivil treatment: H2O2 with Mdivil at 25 μ
m
(P = 0.02) and 50 μ
m
(P = 0.01) concentrations, and 4-hydroxy-2-nonenol with Mdivil at 25 μ
m
(P = 0.03) and 50 μ
m
(P = 0.01) concentrations. In contrast, significantly increased levels were found in the following parameters upon Mdivil treatment: ATP production at 25 μ
m
(P = 0.02) and 50 μ
m
(P = 0.04) concentrations, cell viability at 25 μ
m
(P = 0.04) and 50 μ
m
(P = 0.04) concentrations and GTPase Drp1 activity at 25 μ
m
(P = 0.01) and 50 μ
m
(P = 0.04) concentrations.
Similar articles
- Mitochondria-targeted molecules MitoQ and SS31 reduce mutant huntingtin-induced mitochondrial toxicity and synaptic damage in Huntington's disease.
Yin X, Manczak M, Reddy PH. Yin X, et al. Hum Mol Genet. 2016 May 1;25(9):1739-53. doi: 10.1093/hmg/ddw045. Epub 2016 Feb 16. Hum Mol Genet. 2016. PMID: 26908605 Free PMC article. - Abnormal mitochondrial dynamics, mitochondrial loss and mutant huntingtin oligomers in Huntington's disease: implications for selective neuronal damage.
Shirendeb U, Reddy AP, Manczak M, Calkins MJ, Mao P, Tagle DA, Reddy PH. Shirendeb U, et al. Hum Mol Genet. 2011 Apr 1;20(7):1438-55. doi: 10.1093/hmg/ddr024. Epub 2011 Jan 21. Hum Mol Genet. 2011. PMID: 21257639 Free PMC article. - Mitochondria-Division Inhibitor 1 Protects Against Amyloid-β induced Mitochondrial Fragmentation and Synaptic Damage in Alzheimer's Disease.
Reddy PH, Manczak M, Yin X. Reddy PH, et al. J Alzheimers Dis. 2017;58(1):147-162. doi: 10.3233/JAD-170051. J Alzheimers Dis. 2017. PMID: 28409745 Free PMC article. - Mutant huntingtin, abnormal mitochondrial dynamics, defective axonal transport of mitochondria, and selective synaptic degeneration in Huntington's disease.
Reddy PH, Shirendeb UP. Reddy PH, et al. Biochim Biophys Acta. 2012 Feb;1822(2):101-10. doi: 10.1016/j.bbadis.2011.10.016. Epub 2011 Nov 4. Biochim Biophys Acta. 2012. PMID: 22080977 Free PMC article. Review. - Mitochondria in Huntington's disease.
Damiano M, Galvan L, Déglon N, Brouillet E. Damiano M, et al. Biochim Biophys Acta. 2010 Jan;1802(1):52-61. doi: 10.1016/j.bbadis.2009.07.012. Epub 2009 Aug 11. Biochim Biophys Acta. 2010. PMID: 19682570 Review.
Cited by
- DRP1 Inhibition Rescues Mitochondrial Integrity and Excessive Apoptosis in CS-A Disease Cell Models.
Pascucci B, Spadaro F, Pietraforte D, Nuccio C, Visentin S, Giglio P, Dogliotti E, D'Errico M. Pascucci B, et al. Int J Mol Sci. 2021 Jul 1;22(13):7123. doi: 10.3390/ijms22137123. Int J Mol Sci. 2021. PMID: 34281194 Free PMC article. - Distinct functions of dynamin isoforms in tumorigenesis and their potential as therapeutic targets in cancer.
Meng J. Meng J. Oncotarget. 2017 Jun 20;8(25):41701-41716. doi: 10.18632/oncotarget.16678. Oncotarget. 2017. PMID: 28402939 Free PMC article. Review. - Interactions of amyloidogenic proteins with mitochondrial protein import machinery in aging-related neurodegenerative diseases.
Reed AL, Mitchell W, Alexandrescu AT, Alder NN. Reed AL, et al. Front Physiol. 2023 Nov 2;14:1263420. doi: 10.3389/fphys.2023.1263420. eCollection 2023. Front Physiol. 2023. PMID: 38028797 Free PMC article. Review. - Reduced dynamin-related protein 1 protects against phosphorylated Tau-induced mitochondrial dysfunction and synaptic damage in Alzheimer's disease.
Kandimalla R, Manczak M, Fry D, Suneetha Y, Sesaki H, Reddy PH. Kandimalla R, et al. Hum Mol Genet. 2016 Nov 15;25(22):4881-4897. doi: 10.1093/hmg/ddw312. Hum Mol Genet. 2016. PMID: 28173111 Free PMC article. - Mitochondrial fission drives neuronal metabolic burden to promote stress susceptibility in male mice.
Dong WT, Long LH, Deng Q, Liu D, Wang JL, Wang F, Chen JG. Dong WT, et al. Nat Metab. 2023 Dec;5(12):2220-2236. doi: 10.1038/s42255-023-00924-6. Epub 2023 Nov 20. Nat Metab. 2023. PMID: 37985735
References
- Vonsattel J.P., Myers R.H., Stevens T.J., Ferrante R.J., Bird E.D., Richardson E.P. Jr (1985) Neuropathological classification of Huntington's disease. J. Neuropathol. Exp. Neurol., 44, 559–577. - PubMed
- Folstein S.E. (1990) Huntington's Disease. Johns Hopkins University Press, Baltimore.
- Bates G.P. (2005) History of genetic disease: the molecular genetics of Huntington disease - a history. Nat. Rev. Genet., 6, 766–773. - PubMed
- Politis M., Pavese N., Tai Y.F., Tabrizi S.J., Barker R.A., Piccini P. (2008) Hypothalamic involvement in Huntington's disease: an in vivo PET study. Brain, 131, 2860–2869. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases