A physical perspective on cytoplasmic streaming - PubMed (original) (raw)
A physical perspective on cytoplasmic streaming
Raymond E Goldstein et al. Interface Focus. 2015.
Abstract
Organisms show a remarkable range of sizes, yet the dimensions of a single cell rarely exceed 100 µm. While the physical and biological origins of this constraint remain poorly understood, exceptions to this rule give valuable insights. A well-known counterexample is the aquatic plant Chara, whose cells can exceed 10 cm in length and 1 mm in diameter. Two spiralling bands of molecular motors at the cell periphery drive the cellular fluid up and down at speeds up to 100 µm s(-1), motion that has been hypothesized to mitigate the slowness of metabolite transport on these scales and to aid in homeostasis. This is the most organized instance of a broad class of continuous motions known as 'cytoplasmic streaming', found in a wide range of eukaryotic organisms-algae, plants, amoebae, nematodes and flies-often in unusually large cells. In this overview of the physics of this phenomenon, we examine the interplay between streaming, transport and cell size and discuss the possible role of self-organization phenomena in establishing the observed patterns of streaming.
Keywords: cell size; cytoplasmic streaming; transport.
Figures
Figure 1.
Molecular crowding in eukaryotic cytoplasm. Shown is an illustration of the contents of the yeast Saccharomyces cerevisiae. Proteins, ribosomes with mRNA, microtubules, actin filaments and intermediate filaments are all drawn to scale and at physiological concentrations. (Adapted with permission from [18].)
Figure 2.
Topologies of cytoplasmic streaming. (a) Rotational streaming in internodal cells of Chara corallina. (b) A developing oocyte of Drosophila exhibits a correlated random flow field with typical flow rates of a few to tens of nanometres per second. Schematic of the velocity field, extracted by particle image velocimetry. (c) Circulation streaming in the periphery and transvacuolar strand of epidermal cells, e.g. as found in the root of Medicago truncatula [72]. (d) Reverse-fountain streaming in Lilium longiflorum (left) and Nicotiana tabacum (right). (Image modified from [73].) (e) Periodic shuttle streaming in plasmodium fragments of Physarum polycephalum. (Adapted from [63].)
Figure 3.
Rotational streaming in the characean algae. (a) A shoot of Chara corallina anchored in agar. Single-celled internodes connect nodal complexes where a whorl of six branchlets is formed. (b) Cytoplasmic streaming takes place along two domains shaped as spiralling bands. (c) This circulation is driven by the motion of myosin molecular motors along bundled actin filaments. This image shows a merged stack of confocal slices, with the colours denoting the focal position. Actin bundles can be observed below chloroplast rows at the surface of the cell. (Image courtesy S. Ganguly.) (d) The motion of myosin at the periphery entrains the outer layer of cytoplasm, which is of order 10 µm in thickness. The two moving bands are separated by a neutral line visible as a row of missing chloroplasts. The motion at the wall induces a shear flow in the central vacuole of the cell.
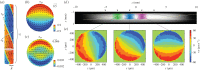
Figure 4.
Hydrodynamic prediction and MRV measurements of vacuolar flow. (a) The internodal flow has a helical symmetry: an invariance under a translation along the longitudinal axis combined with a rotation. The two axes that naturally follow from this symmetry are the vector e H, which points along the bands, and the vector e φ , which is orthogonal to the bands. (b) Theoretically predicted flow field along the e H component, showing the vacuolar shear profile. (c) The e r and e φ components reveal a small secondary circulation along the centre of the cell. (d) MRI scan of an internode placed in a glass tube, with spiralling lines indicating positions of the neutral line, and coloured bands showing the domains used for velocity measurements. (e) Velocity profiles measured at each of the domains show excellent agreement with the theoretical profile. (Figures modified from [–116].)
Figure 5.
Self-organization of cytoplasmic streaming in a mathematical model of Chara [174]. Colour coding corresponds to the _z_-component of an order parameter associated with actin filaments at the periphery, and white lines represent indifferent zones separating up- and down-streaming regions. Superimposed are streamlines of the cytoplasmic flow induced by the filament field, where the flow is directed from the thin end to the thick end of the individual lines. Panels show progression from random disorder through local order to complete steady cyclosis.
Similar articles
- Microfluidics of cytoplasmic streaming and its implications for intracellular transport.
Goldstein RE, Tuval I, van de Meent JW. Goldstein RE, et al. Proc Natl Acad Sci U S A. 2008 Mar 11;105(10):3663-7. doi: 10.1073/pnas.0707223105. Epub 2008 Feb 29. Proc Natl Acad Sci U S A. 2008. PMID: 18310326 Free PMC article. - Go with the flow - bulk transport by molecular motors.
Lu W, Gelfand VI. Lu W, et al. J Cell Sci. 2023 Mar 1;136(5):jcs260300. doi: 10.1242/jcs.260300. Epub 2022 Oct 17. J Cell Sci. 2023. PMID: 36250267 Free PMC article. Review. - Cytoplasmic streaming in plant cells emerges naturally by microfilament self-organization.
Woodhouse FG, Goldstein RE. Woodhouse FG, et al. Proc Natl Acad Sci U S A. 2013 Aug 27;110(35):14132-7. doi: 10.1073/pnas.1302736110. Epub 2013 Aug 12. Proc Natl Acad Sci U S A. 2013. PMID: 23940314 Free PMC article. - Mechanosensing and anesthesia of single internodal cells of Chara.
Rodgers MJ, Staves MP. Rodgers MJ, et al. Plant Signal Behav. 2024 Dec 31;19(1):2339574. doi: 10.1080/15592324.2024.2339574. Epub 2024 Apr 11. Plant Signal Behav. 2024. PMID: 38601988 Free PMC article. - Cytoplasmic streaming enables the distribution of molecules and vesicles in large plant cells.
Verchot-Lubicz J, Goldstein RE. Verchot-Lubicz J, et al. Protoplasma. 2010 Apr;240(1-4):99-107. doi: 10.1007/s00709-009-0088-x. Epub 2009 Nov 25. Protoplasma. 2010. PMID: 19937356 Review.
Cited by
- A novel mechanism of bulk cytoplasmic transport by cortical dynein in Drosophila ovary.
Lu W, Lakonishok M, Serpinskaya AS, Gelfand VI. Lu W, et al. Elife. 2022 Feb 16;11:e75538. doi: 10.7554/eLife.75538. Elife. 2022. PMID: 35170428 Free PMC article. - Sustained enzymatic activity and flow in crowded protein droplets.
Testa A, Dindo M, Rebane AA, Nasouri B, Style RW, Golestanian R, Dufresne ER, Laurino P. Testa A, et al. Nat Commun. 2021 Nov 1;12(1):6293. doi: 10.1038/s41467-021-26532-0. Nat Commun. 2021. PMID: 34725341 Free PMC article. - Differentiation Disorders of Chara vulgaris Spermatids following Treatment with Propyzamide.
Wojtczak A. Wojtczak A. Cells. 2023 Apr 27;12(9):1268. doi: 10.3390/cells12091268. Cells. 2023. PMID: 37174667 Free PMC article. - Active membrane deformations of a minimal synthetic cell.
Sciortino A, Faizi HA, Fedosov DA, Frechette L, Vlahovska PM, Gompper G, Bausch AR. Sciortino A, et al. Nat Phys. 2025;21(5):799-807. doi: 10.1038/s41567-025-02839-3. Epub 2025 Mar 24. Nat Phys. 2025. PMID: 40386801 Free PMC article. - Biomolecular condensates undergo a generic shear-mediated liquid-to-solid transition.
Shen Y, Ruggeri FS, Vigolo D, Kamada A, Qamar S, Levin A, Iserman C, Alberti S, George-Hyslop PS, Knowles TPJ. Shen Y, et al. Nat Nanotechnol. 2020 Oct;15(10):841-847. doi: 10.1038/s41565-020-0731-4. Epub 2020 Jul 13. Nat Nanotechnol. 2020. PMID: 32661370 Free PMC article.
References
- Cannon WB. 1932. The wisdom of the body. New York, NY: WW. Norton & Co., Inc.
- Boller T, Wiemken A. 1986. Dynamics of vacuolar compartmentation. Annu. Rev. Plant Physiol. 37, 137–164. (10.1146/annurev.pp.37.060186.001033) - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources