FetA Antibodies Induced by an Outer Membrane Vesicle Vaccine Derived from a Serogroup B Meningococcal Isolate with Constitutive FetA Expression - PubMed (original) (raw)
FetA Antibodies Induced by an Outer Membrane Vesicle Vaccine Derived from a Serogroup B Meningococcal Isolate with Constitutive FetA Expression
Holly Sanders et al. PLoS One. 2015.
Abstract
Invasive meningococcal disease causes over 3500 cases each year in Europe, with particularly high incidence among young children. Among serogroup B meningococci, which cause most of the cases, high diversity in the outer membrane proteins (OMPs) is observed in endemic situations; however, comprehensive molecular epidemiological data are available for the diversity and distribution of the OMPs PorA and FetA and these can be used to rationally design a vaccine with high coverage of the case isolates. The aim of this study was to determine whether outer membrane vesicles (OMVs) derived from an isolate with constitutive FetA expression (MenPF-1 vaccine) could be used to induce antibodies against both the PorA and FetA antigens. The immunogenicity of various dose levels and number of doses was evaluated in mice and rabbits, and IgG antibody responses tested against OMVs and recombinant PorA and FetA proteins. A panel of four isogenic mutants was generated and used to evaluate the relative ability of the vaccine to induce serum bactericidal activity (SBA) against FetA and PorA. Sera from mice were tested in SBA against the four target strains. Results demonstrated that the MenPF-1 OMVs were immunogenic against PorA and FetA in both animal models. Furthermore, the murine antibodies induced were bactericidal against isogenic mutant strains, suggesting that antibodies to both PorA and FetA were functional. The data presented indicate that the MenPF-1 vaccine is a suitable formulation for presenting PorA and FetA OMPs in order to induce bactericidal antibodies, and that proceeding to a Phase I clinical trial with this vaccine candidate is justified.
Conflict of interest statement
Competing Interests: The authors have read the journal's policy and have the following competing interests: HS is currently an employee of Janssen Infectious Disease and Vaccines but had no affiliation to this company at the time of conducting the work presented in the manuscript. AP, JP and MM are named inventors on a patent application concerning a PorA FetA vaccine but this application is no longer being pursued. AP has previously led clinical trials of meningococcal vaccines on behalf of Oxford University sponsored by Novartis and Pfizer but no longer does so and does not receive any personal payments or travel funds from vaccine manufacturers. AP chairs the Department of Health’s (DH) Joint Committee on Vaccination and Immunisation (JCVI) but the views expressed do not express the views of DH or JCVI. On behalf of Isis Innovations Ltd., a subsidiary owned by the University of Oxford, MM undertakes occasional consultancies in the area of meningococcal vaccines and population biology for the pharmaceutical industry. The Wellcome Trust has been involved in the decision to publish this work and critical review of the manuscript. All other authors have declared that no competing interests exist. This does not alter the authors' adherence to PLOS ONE policies on sharing data and materials.
Figures
Fig 1. Sequence of the modified porA promoter region used to replace the native fetA promoter.
Bases constituting the -10 and -35 regions are highlighted in green. Bases taken from the porB promoter are highlighted in blue.
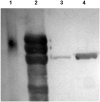
Fig 2. Verification of FetA expression in wildtype (H44/76) and genetically modified strain (SMenPF1.2).
Analysis by immunoblot (antiserum raised to recombinant FetA variant F3-3) of SDS-PAGE gel with 10 μg protein (outer membrane protein preparations) per well. BioRad pre-stained SDS broad-range standards (70kDa standard shown in Lane 1). Lane 2: Purified FetA F3-3. Lane 3: Wildtype H44/76. Lane 4: Constitutive Strain (SMenPF1.2).
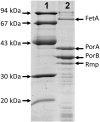
Fig 3. Characterisation by SDS-PAGE of the MenPF1 outer membrane vesicles used for immunization.
Lanes: 1) Molecular weight protein markers. 2) OMVs from strain SMenPF1.2.
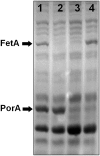
Fig 4. SDS-PAGE of outer membrane proteins from the isogenic mutant strains used to evaluate the serum bactericidal response.
50 μg total protein was loaded per lane. Lanes: 1) SMenPF1.2 (fetAp 17bp), 2) 3043 (fetA::kan), 3) 3311 (fetA::kan porA::ermC), 4) 3312 (fetAp 17bp porA::ermC).
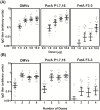
Fig 5. Immunogenicity of MenPF-1 vaccine in mice.
Total IgG titres, calculated in comparison to a pooled standard serum, were determined for sera from individual mice against bulk OMVs and the PorA and FetA antigens. The standard serum was given an arbitrary value of 10 units. The geometric mean IgG titres are indicated with a line. Error bars show the 95% confidence intervals of the mean. (A) Mice given two doses of four dose levels or an μg protein per doses. Note that the data from mice given two doses of 2.5 μg protein per dose is included in both figures for comparison, as the immunizations were performed concomitantly. Actual figures for the data presented are given in S1 Table.
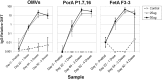
Fig 6. Immunogenicity of strain MenPF-1 in rabbits.
Total IgG titres calculated in comparison to a pooled standard serum, were determined for sera from individual rabbits against bulk OMVs and the PorA P1.7,16 and FetA F3-3 antigens. The standard serum was given an arbitrary value of 10 units. Rabbits were immunized with four doses of either adjuvant alone (Control), 25 μg total protein per doses, or 50 μg total protein per doses. Blood samples were collected from all animals (n = 12) pretrial (Sample 1), and before dosing on days 22 (Sample 2, post one dose) and 64 (Sample 3, post three doses). A fourth blood sample was taken from six rabbits on day 92 (Sample 4, post four doses). The graph shows geometric mean units. Error bars indicate the 95% confidence intervals of the mean. Actual figures for the data presented are given in S2 Table.
Similar articles
- A novel meningococcal outer membrane vesicle vaccine with constitutive expression of FetA: A phase I clinical trial.
Marsay L, Dold C, Green CA, Rollier CS, Norheim G, Sadarangani M, Shanyinde M, Brehony C, Thompson AJ, Sanders H, Chan H, Haworth K, Derrick JP, Feavers IM, Maiden MC, Pollard AJ. Marsay L, et al. J Infect. 2015 Sep;71(3):326-37. doi: 10.1016/j.jinf.2015.05.006. Epub 2015 May 15. J Infect. 2015. PMID: 25982025 Free PMC article. Clinical Trial. - An OMV Vaccine Derived from a Capsular Group B Meningococcus with Constitutive FetA Expression: Preclinical Evaluation of Immunogenicity and Toxicity.
Norheim G, Sanders H, Mellesdal JW, Sundfør I, Chan H, Brehony C, Vipond C, Dold C, Care R, Saleem M, Maiden MC, Derrick JP, Feavers I, Pollard AJ. Norheim G, et al. PLoS One. 2015 Sep 21;10(9):e0134353. doi: 10.1371/journal.pone.0134353. eCollection 2015. PLoS One. 2015. PMID: 26390123 Free PMC article. - Deletion of major porins from meningococcal outer membrane vesicle vaccines enhances reactivity against heterologous serogroup B Neisseria meningitidis strains.
Matthias KA, Reveille A, Connolly KL, Jerse AE, Gao YS, Bash MC. Matthias KA, et al. Vaccine. 2020 Feb 28;38(10):2396-2405. doi: 10.1016/j.vaccine.2020.01.038. Epub 2020 Feb 7. Vaccine. 2020. PMID: 32037226 Free PMC article. - The Development of a Vaccine Against Meningococcus B Using Reverse Vaccinology.
Masignani V, Pizza M, Moxon ER. Masignani V, et al. Front Immunol. 2019 Apr 16;10:751. doi: 10.3389/fimmu.2019.00751. eCollection 2019. Front Immunol. 2019. PMID: 31040844 Free PMC article. Review. - Multivalent meningococcal serogroup B vaccines: challenges in predicting protection and measuring effectiveness.
Poolman JT, Richmond P. Poolman JT, et al. Expert Rev Vaccines. 2015;14(9):1277-87. doi: 10.1586/14760584.2015.1071670. Epub 2015 Jul 23. Expert Rev Vaccines. 2015. PMID: 26204792 Review.
Cited by
- Human B Cell Responses to Dominant and Subdominant Antigens Induced by a Meningococcal Outer Membrane Vesicle Vaccine in a Phase I Trial.
Rollier CS, Dold C, Marsay L, Linder A, Green CA, Sadarangani M, Norheim G, Derrick JP, Feavers IM, Maiden MCJ, Pollard AJ. Rollier CS, et al. mSphere. 2022 Feb 23;7(1):e0067421. doi: 10.1128/msphere.00674-21. Epub 2022 Jan 26. mSphere. 2022. PMID: 35080470 Free PMC article. Clinical Trial. - Cell membrane-derived nanomaterials for biomedical applications.
Fang RH, Jiang Y, Fang JC, Zhang L. Fang RH, et al. Biomaterials. 2017 Jun;128:69-83. doi: 10.1016/j.biomaterials.2017.02.041. Epub 2017 Mar 1. Biomaterials. 2017. PMID: 28292726 Free PMC article. Review. - Immunogenicity profiling of protein antigens from capsular group B Neisseria meningitidis.
Awanye AM, Chang CM, Wheeler JX, Chan H, Marsay L, Dold C, Rollier CS, Bird LE, Nettleship JE, Owens RJ, Pollard AJ, Derrick JP. Awanye AM, et al. Sci Rep. 2019 May 2;9(1):6843. doi: 10.1038/s41598-019-43139-0. Sci Rep. 2019. PMID: 31048732 Free PMC article. Clinical Trial. - Outer membrane vesicles as a platform for the discovery of antibodies to bacterial pathogens.
Lei EK, Azmat A, Henry KA, Hussack G. Lei EK, et al. Appl Microbiol Biotechnol. 2024 Feb 24;108(1):232. doi: 10.1007/s00253-024-13033-5. Appl Microbiol Biotechnol. 2024. PMID: 38396192 Free PMC article. Review. - A novel meningococcal outer membrane vesicle vaccine with constitutive expression of FetA: A phase I clinical trial.
Marsay L, Dold C, Green CA, Rollier CS, Norheim G, Sadarangani M, Shanyinde M, Brehony C, Thompson AJ, Sanders H, Chan H, Haworth K, Derrick JP, Feavers IM, Maiden MC, Pollard AJ. Marsay L, et al. J Infect. 2015 Sep;71(3):326-37. doi: 10.1016/j.jinf.2015.05.006. Epub 2015 May 15. J Infect. 2015. PMID: 25982025 Free PMC article. Clinical Trial.
References
- ECDC (2013) Annual Epidemiological Report 2012: Reporting on 2010 surveillance data and 2011 epidemic intelligence data. Stockholm: ECDC.
- Yongye AB, Gonzalez-Outeirino J, Glushka J, Schultheis V, Woods RJ (2008) The conformational properties of methyl alpha-(2,8)-di/trisialosides and their N-acyl analogues: implications for anti-Neisseria meningitidis B vaccine design. Biochemistry 47: 12493–12514. 10.1021/bi800431c - DOI - PMC - PubMed
- Finne J, Leinonen M, Makela PH (1983) Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet 2: 355–357. - PubMed
- Holst J (2007) Strategies for development of universal vaccines against meningococcal serogroup B disease: the most promising options and the challenges evaluating them. Hum Vaccin 3: 290–294. - PubMed
- Sierra GV, Campa HC, Varcacel NM, Garcia IL, Izquierdo PL, Sotolongo PF, et al. (1991) Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann 14: 195–207; discussion 208–110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources