Proton Channel Activity of Influenza A Virus Matrix Protein 2 Contributes to Autophagy Arrest - PubMed (original) (raw)
. 2015 Oct 14;90(1):591-8.
doi: 10.1128/JVI.00576-15. Print 2016 Jan 1.
Chufang Li 2, Liqiang Feng 3, Weiqi Pan 4, Liang Li 4, Qian Wang 3, Jiashun Li 4, Na Li 4, Ling Han 4, Xuehua Zheng 4, Xuefeng Niu 4, Caijun Sun 3, Ling Chen 5
Affiliations
- PMID: 26468520
- PMCID: PMC4702553
- DOI: 10.1128/JVI.00576-15
Proton Channel Activity of Influenza A Virus Matrix Protein 2 Contributes to Autophagy Arrest
Yizhong Ren et al. J Virol. 2015.
Abstract
Influenza A virus infection can arrest autophagy, as evidenced by autophagosome accumulation in infected cells. Here, we report that this autophagosome accumulation can be inhibited by amantadine, an antiviral proton channel inhibitor, in amantadine-sensitive virus infected cells or cells expressing influenza A virus matrix protein 2 (M2). Thus, M2 proton channel activity plays a role in blocking the fusion of autophagosomes with lysosomes, which might be a key mechanism for arresting autophagy.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
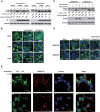
FIG 1
Inhibition of proton channel activity attenuates influenza A virus M2-induced autophagosome accumulation. (A) HEK293 cells were infected with influenza virus A/Hong Kong/8/68 (H3N2) or A/Wisconsin/33(H1N1) at an MOI of 5. Amantadine (5 μM) or oseltamivir (200 nM) was added at 3 h after infection. Cell were collected at 12 h after infection and subjected to Western blot analysis with the antibodies indicated. NP, nucleoprotein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. (B) HEK293 cells with stable expression of GFP-LC3 were treated as described for panel A and observed by confocal microscopy. Scale bars, 20 μm. (C) TREx-293 cells carrying tetracycline-inducible amantadine-sensitive H5N1-M2 (S31) or amantadine-resistant H5N1-M2 (N31) were treated with tetracycline (1 μg/ml) with or without amantadine. Cells were collected at 24 h and subjected to Western blot analysis with the antibodies indicated. (D) GFP-LC3-transfected TREx-293 cells carrying tetracycline-inducible H5N1-M2 (S31) or H5N1-M2 (N31) were treated with tetracycline with or without amantadine. Cells were observed 24 h later by confocal microscopy. Scale bars, 20 μm. (E) GFP-LC3-transfected TREx-293 cells carrying tetracycline-inducible amantadine-sensitive H5N1-M2 (S31) were treated with tetracycline with or without amantadine for 24 h. Cells were weakly permeabilized for M2 immunostaining and observed by confocal microscopy. Scale bars, 10 μm. (F, G) MDCK (F) or MCF-7 (G) cells stably expressing GFP-LC3 were transfected, respectively, with plasmids expressing H5M2(S31). Amantadine was added at 6 h after transfection. Cells were collected at 24 h and subjected to immunofluorescence staining with anti-M2 antibodies and a DyLight 549-labeled goat anti-mouse secondary antibody. The cells were observed by fluorescence microscopy. Scale bars, 20 μm. (H) HEK293 cells were transfected with plasmid pcDNA4, pcDNA4-H7N9-M2 (S31), or pcDNA4-H7N9-M2 (N31) and then treated with or without amantadine at 6 h after transfection. Cell were collected at 24 h after amantadine treatment and subjected to Western blot analysis with the antibodies indicated. (I) HEK293 cells with stable expression of GFP-LC3 were transfected with plasmid pcDNA4, pcDNA4-H7N9-M2 (S31), or pcDNA4-H7N9-M2 (N31) and then treated with amantadine at 6 h after transfection. Cells were observed by confocal microscopy at 24 h after amantadine treatment. Scale bars, 20 μm. Representative data from one of three separate experiments are shown. The relative LC3-II/LC3-I, LC3-II/GADPH, and P62/GADPH ratios were analyzed with ImageJ (National Institutes of Health) in the same Western blot assay. The anti-M2 mouse serum was generated in our lab, the anti-influenza A virus nucleoprotein antibody was Abcam Ab139361, the anti-LC3-II antibody was Sigma-Aldrich L7543, and the anti-P62 antibody was Sigma-Aldrich P0067.
FIG 1
Inhibition of proton channel activity attenuates influenza A virus M2-induced autophagosome accumulation. (A) HEK293 cells were infected with influenza virus A/Hong Kong/8/68 (H3N2) or A/Wisconsin/33(H1N1) at an MOI of 5. Amantadine (5 μM) or oseltamivir (200 nM) was added at 3 h after infection. Cell were collected at 12 h after infection and subjected to Western blot analysis with the antibodies indicated. NP, nucleoprotein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. (B) HEK293 cells with stable expression of GFP-LC3 were treated as described for panel A and observed by confocal microscopy. Scale bars, 20 μm. (C) TREx-293 cells carrying tetracycline-inducible amantadine-sensitive H5N1-M2 (S31) or amantadine-resistant H5N1-M2 (N31) were treated with tetracycline (1 μg/ml) with or without amantadine. Cells were collected at 24 h and subjected to Western blot analysis with the antibodies indicated. (D) GFP-LC3-transfected TREx-293 cells carrying tetracycline-inducible H5N1-M2 (S31) or H5N1-M2 (N31) were treated with tetracycline with or without amantadine. Cells were observed 24 h later by confocal microscopy. Scale bars, 20 μm. (E) GFP-LC3-transfected TREx-293 cells carrying tetracycline-inducible amantadine-sensitive H5N1-M2 (S31) were treated with tetracycline with or without amantadine for 24 h. Cells were weakly permeabilized for M2 immunostaining and observed by confocal microscopy. Scale bars, 10 μm. (F, G) MDCK (F) or MCF-7 (G) cells stably expressing GFP-LC3 were transfected, respectively, with plasmids expressing H5M2(S31). Amantadine was added at 6 h after transfection. Cells were collected at 24 h and subjected to immunofluorescence staining with anti-M2 antibodies and a DyLight 549-labeled goat anti-mouse secondary antibody. The cells were observed by fluorescence microscopy. Scale bars, 20 μm. (H) HEK293 cells were transfected with plasmid pcDNA4, pcDNA4-H7N9-M2 (S31), or pcDNA4-H7N9-M2 (N31) and then treated with or without amantadine at 6 h after transfection. Cell were collected at 24 h after amantadine treatment and subjected to Western blot analysis with the antibodies indicated. (I) HEK293 cells with stable expression of GFP-LC3 were transfected with plasmid pcDNA4, pcDNA4-H7N9-M2 (S31), or pcDNA4-H7N9-M2 (N31) and then treated with amantadine at 6 h after transfection. Cells were observed by confocal microscopy at 24 h after amantadine treatment. Scale bars, 20 μm. Representative data from one of three separate experiments are shown. The relative LC3-II/LC3-I, LC3-II/GADPH, and P62/GADPH ratios were analyzed with ImageJ (National Institutes of Health) in the same Western blot assay. The anti-M2 mouse serum was generated in our lab, the anti-influenza A virus nucleoprotein antibody was Abcam Ab139361, the anti-LC3-II antibody was Sigma-Aldrich L7543, and the anti-P62 antibody was Sigma-Aldrich P0067.
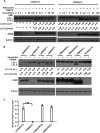
FIG 2
Proton channel activity contributes to influenza A virus M2-induced autophagosome accumulation in a dose-dependent manner. (A) TREx-293 cells carrying tetracycline-inducible amantadine-sensitive H5N1-M2 (S31) or amantadine-resistant H5N1-M2 (N31) were treated and with increasing concentrations of amantadine with or without tetracycline as indicated. Cells were collected at 24 h and subjected to Western blot analysis with the antibodies indicated. (B) HEK293 cells were transfected with plasmids expressing H5M2 (S31), H5M2 (N31), H5M2 (H37G), H5M2 (HDR/A), or H5M2 (HDR/L). Amantadine was added at 6 h after transfection. Cells were collected at 24 h and subjected to Western blot analysis with the antibodies indicated. Representative data from one of three separate experiments are shown. (C) MDCK cells were transfected with plasmids expressing H5M2 (S31), H5M2 (HDR/A), or H5M2 (HDR/L) with a pH-sensitive EGFP protein in the wells of a 96-well plate in six replicates. At 16 h after transfection, the culture medium was replaced with pH 7.4 phosphate-buffered saline containing 0.3% bovine serum albumin for detection of fluorescence intensity at neutral pH. The change in fluorescence intensity was detected at 1 min after the medium was changed to pH 5.0. The relative fluorescence was the ratio of fluorescence intensity at pH 5.0 normalized to that at pH 7.4. Statistical analyses were performed by two-tailed Mann-Whitney test. ***, P < 0.001. Representative data from one of three separate experiments are shown. The relative LC3-II/LC3-I and LC3-II/β-actin ratios were analyzed by using ImageJ (National Institutes of Health) in the same Western blot assay. M2(HDR/A) has His37Ala, Asp44Ala, and Arg45Ala mutations. M2(HDR/L) has His37Leu, Asp44 Leu, and Arg45Leu mutations.
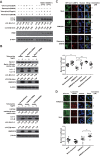
FIG 3
Proton channel activity of influenza A virus M2 is required to block the fusion of autophagosomes with lysosomes. (A)TREx-293 cells carrying tetracycline-inducible H5N1-M2 (S31) or H5N1-M2 (N31) were treated with different compounds as indicated. Cells were collected at 24 h and subjected to Western blot analysis. (B) TREx-293 cells carrying tetracycline-inducible H5N1-M2 (S31) were transfected with siRNA for Beclin 1 (Atg6), hVPS34, or negative siRNA as a control (purchased from RiboBio). Twenty-four hours later, cells were treated with 1 μg/ml tetracycline for another 24 h. Cell lysates were subjected to Western blot analysis with the antibodies indicated (anti-Beclin 1 [Sigma B6186] and anti-PI3K hVPS34 [Cell Signaling 3358] antibodies). (C) HEK293 cells stably expressing GFP-LC3 were either transfected with a plasmid encoding H5N1-M2 (S31) or treated with rapamycin for 24 h. Amantadine was then added for another 3 h of incubation. Cells were stained with an anti-LAMP2 antibody (Abcam Ab18529) and a Cy3-conjugated goat anti-rabbit secondary antibody and observed by confocal microscopy. Scale bars, 20 μm. (D) GFP-LC3-transfected TREx-293 cells with tetracycline-inducible H5N1-M2 (S31) were treated with tetracycline for 24 h and then treated with LysoTracker Red DND99 (Life Technologies L7528) for 2 h and amantadine for 1 h. The cells were observed by fluorescence microscopy. Scale bars, 20 μm. The colocalization of GFP-LC3 with the lysosome marker LAMP2 or LysoTracker Red DND99 was analyzed with Image Pro (Media Cybernetics). Overlap is indicated by Manders' overlap coefficients ranging from 0 to 1. A value of 1 implies that 100% of both selected channels is colocalized. A total of 10 cells were analyzed. Statistical analyses were performed by two-tailed Mann-Whitney test. **, P < 0.01; ***, P < 0.001. Representative data from one of three separate experiments are shown. Relative LC3-II/LC3-I, LC3-II/β-actin, Beclin 1/β-actin, and hVPS34/β-actin ratios were analyzed by using ImageJ (National Institutes of Health) in the same Western blot assay.
Similar articles
- Salinomycin Inhibits Influenza Virus Infection by Disrupting Endosomal Acidification and Viral Matrix Protein 2 Function.
Jang Y, Shin JS, Yoon YS, Go YY, Lee HW, Kwon OS, Park S, Park MS, Kim M. Jang Y, et al. J Virol. 2018 Nov 27;92(24):e01441-18. doi: 10.1128/JVI.01441-18. Print 2018 Dec 15. J Virol. 2018. PMID: 30282713 Free PMC article. - Viral M2 ion channel protein: a promising target for anti-influenza drug discovery.
Moorthy NS, Poongavanam V, Pratheepa V. Moorthy NS, et al. Mini Rev Med Chem. 2014;14(10):819-30. Mini Rev Med Chem. 2014. PMID: 25342196 Review. - Role of virion M2 protein in influenza virus uncoating: specific reduction in the rate of membrane fusion between virus and liposomes by amantadine.
Wharton SA, Belshe RB, Skehel JJ, Hay AJ. Wharton SA, et al. J Gen Virol. 1994 Apr;75 ( Pt 4):945-8. doi: 10.1099/0022-1317-75-4-945. J Gen Virol. 1994. PMID: 8151308 - A LC3-interacting motif in the influenza A virus M2 protein is required to subvert autophagy and maintain virion stability.
Beale R, Wise H, Stuart A, Ravenhill BJ, Digard P, Randow F. Beale R, et al. Cell Host Microbe. 2014 Feb 12;15(2):239-47. doi: 10.1016/j.chom.2014.01.006. Cell Host Microbe. 2014. PMID: 24528869 Free PMC article. - [Structure and function of the influenza virus M2 ion channel protein].
Sakaguchi T. Sakaguchi T. Nihon Rinsho. 1997 Oct;55(10):2587-92. Nihon Rinsho. 1997. PMID: 9360376 Review. Japanese.
Cited by
- Autophagy in Negative-Strand RNA Virus Infection.
Wang Y, Jiang K, Zhang Q, Meng S, Ding C. Wang Y, et al. Front Microbiol. 2018 Feb 13;9:206. doi: 10.3389/fmicb.2018.00206. eCollection 2018. Front Microbiol. 2018. PMID: 29487586 Free PMC article. Review. - In Vivo Therapy with M2e-Specific IgG Selects for an Influenza A Virus Mutant with Delayed Matrix Protein 2 Expression.
Van den Hoecke S, Ballegeer M, Vrancken B, Deng L, Job ER, Roose K, Schepens B, Van Hoecke L, Lemey P, Saelens X. Van den Hoecke S, et al. mBio. 2021 Aug 31;12(4):e0074521. doi: 10.1128/mBio.00745-21. Epub 2021 Jul 13. mBio. 2021. PMID: 34253060 Free PMC article. - Influenza A virus protein PB1-F2 impairs innate immunity by inducing mitophagy.
Wang R, Zhu Y, Ren C, Yang S, Tian S, Chen H, Jin M, Zhou H. Wang R, et al. Autophagy. 2021 Feb;17(2):496-511. doi: 10.1080/15548627.2020.1725375. Epub 2020 Feb 11. Autophagy. 2021. PMID: 32013669 Free PMC article. - Sarco/Endoplasmic Reticulum Ca2+ Transporting ATPase (SERCA) Modulates Autophagic, Inflammatory, and Mitochondrial Responses during Influenza A Virus Infection in Human Lung Cells.
Peng J, Ran Y, Xie H, Deng L, Li C, Ling C. Peng J, et al. J Virol. 2021 Apr 26;95(10):e00217-21. doi: 10.1128/JVI.00217-21. Epub 2021 Mar 10. J Virol. 2021. PMID: 33692207 Free PMC article. - The inducible amphisome isolates viral hemagglutinin and defends against influenza A virus infection.
Omi J, Watanabe-Takahashi M, Igai K, Shimizu E, Tseng CY, Miyasaka T, Waku T, Hama S, Nakanishi R, Goto Y, Nishino Y, Miyazawa A, Natori Y, Yamashita M, Nishikawa K. Omi J, et al. Nat Commun. 2020 Jan 9;11(1):162. doi: 10.1038/s41467-019-13974-w. Nat Commun. 2020. PMID: 31919357 Free PMC article.
References
- Li KS, Guan Y, Wang J, Smith GJ, Xu KM, Duan L, Rahardjo AP, Puthavathana P, Buranathai C, Nguyen TD, Estoepangestie AT, Chaisingh A, Auewarakul P, Long HT, Hanh NT, Webby RJ, Poon LL, Chen H, Shortridge KF, Yuen KY, Webster RG, Peiris JS. 2004. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature 430:209–213. doi:10.1038/nature02746. - DOI - PubMed
- Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, Xu X, Lu H, Zhu W, Gao Z, Xiang N, Shen Y, He Z, Gu Y, Zhang Z, Yang Y, Zhao X, Zhou L, Li X, Zou S, Zhang Y, Yang L, Guo J, Dong J, Li Q, Dong L, Zhu Y, Bai T, Wang S, Hao P, Yang W, Han J, Yu H, Li D, Gao GF, Wu G, Wang Y, Yuan Z, Shu Y. 2013. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med 368:1888–1897. doi:10.1056/NEJMoa1304459. - DOI - PubMed
- Shapira SD, Gat-Viks I, Shum BO, Dricot A, de Grace MM, Wu L, Gupta PB, Hao T, Silver SJ, Root DE, Hill DE, Regev A, Hacohen N. 2009. A physical and regulatory map of host-influenza interactions reveals pathways in H1N1 infection. Cell 139:1255–1267. doi:10.1016/j.cell.2009.12.018. - DOI - PMC - PubMed
- Gannagé M, Dormann D, Albrecht R, Dengjel J, Torossi T, Ramer PC, Lee M, Strowig T, Arrey F, Conenello G, Pypaert M, Andersen J, Garcia-Sastre A, Munz C. 2009. Matrix protein 2 of influenza A virus blocks autophagosome fusion with lysosomes. Cell Host Microbe 6:367–380. doi:10.1016/j.chom.2009.09.005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources