Evaluation of the Broad-Range PCR/ESI-MS Technology in Blood Specimens for the Molecular Diagnosis of Bloodstream Infections - PubMed (original) (raw)
Evaluation of the Broad-Range PCR/ESI-MS Technology in Blood Specimens for the Molecular Diagnosis of Bloodstream Infections
Elena Jordana-Lluch et al. PLoS One. 2015.
Abstract
Background: Rapid identification of the etiological agent in bloodstream infections is of vital importance for the early administration of the most appropriate antibiotic therapy. Molecular methods may offer an advantage to current culture-based microbiological diagnosis. The goal of this study was to evaluate the performance of IRIDICA, a platform based on universal genetic amplification followed by mass spectrometry (PCR/ESI-MS) for the molecular diagnosis of sepsis-related pathogens directly from the patient's blood.
Methods: A total of 410 whole blood specimens from patients admitted to Emergency Room (ER) and Intensive Care Unit (ICU) with clinical suspicion of sepsis were tested with the IRIDICA BAC BSI Assay (broad identification of bacteria and Candida spp.). Microorganisms grown in culture and detected by IRIDICA were compared considering blood culture as gold standard. When discrepancies were found, clinical records and results from other cultures were taken into consideration (clinical infection criterion).
Results: The overall positive and negative agreement of IRIDICA with blood culture in the analysis by specimen was 74.8% and 78.6%, respectively, rising to 76.9% and 87.2% respectively, when compared with the clinical infection criterion. Interestingly, IRIDICA detected 41 clinically significant microorganisms missed by culture, most of them from patients under antimicrobial treatment. Of special interest were the detections of one Mycoplasma hominis and two Mycobacterium simiae in immunocompromised patients. When ICU patients were analyzed separately, sensitivity, specificity, positive and negative predictive values compared with blood culture were 83.3%, 78.6%, 33.9% and 97.3% respectively, and 90.5%, 87.2%, 64.4% and 97.3% respectively, in comparison with the clinical infection criterion.
Conclusions: IRIDICA is a promising technology that offers an early and reliable identification of a wide variety of pathogens directly from the patient's blood within 6h, which brings the opportunity to improve management of septic patients, especially for those critically ill admitted to the ICU.
Conflict of interest statement
Competing Interests: EJL received personal fees and travel grants from Abbott Molecular as a speaker in meetings. All reagents and materials necessary for IRIDICA testing were supplied by Ibis Biosciences, an Abbott Molecular company. This commercial funder had no role in study design, data collection and analysis decision to publish, or preparation of the manuscript. This does not alter the authors’ adherence to all the PLOS ONE policies on sharing data and materials.
Figures
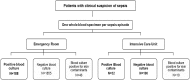
Fig 1. Flowchart depicting the study design.
Similar articles
- Molecular diagnosis of bloodstream infections in onco-haematology patients with PCR/ESI-MS technology.
Jordana-Lluch E, Rivaya B, Marcó C, Giménez M, Quesada MD, Escobedo A, Batlle M, Martró E, Ausina V. Jordana-Lluch E, et al. J Infect. 2017 Feb;74(2):187-194. doi: 10.1016/j.jinf.2016.11.011. Epub 2016 Nov 23. J Infect. 2017. PMID: 27889413 - Rapid diagnosis of bloodstream infections in the critically ill: Evaluation of the broad-range PCR/ESI-MS technology.
Tassinari M, Zannoli S, Farabegoli P, Pedna MF, Pierro A, Mastroianni A, Fontan R, Luongo L, Sarnataro G, Menegatti E, Caruso A, Sambri V. Tassinari M, et al. PLoS One. 2018 May 15;13(5):e0197436. doi: 10.1371/journal.pone.0197436. eCollection 2018. PLoS One. 2018. PMID: 29763469 Free PMC article. - The detection of microbial DNA but not cultured bacteria is associated with increased mortality in patients with suspected sepsis-a prospective multi-centre European observational study.
O'Dwyer MJ, Starczewska MH, Schrenzel J, Zacharowski K, Ecker DJ, Sampath R, Brealey D, Singer M, Libert N, Wilks M, Vincent JL. O'Dwyer MJ, et al. Clin Microbiol Infect. 2017 Mar;23(3):208.e1-208.e6. doi: 10.1016/j.cmi.2016.11.010. Epub 2016 Nov 23. Clin Microbiol Infect. 2017. PMID: 27890455 - Microbial diagnosis of bloodstream infection: towards molecular diagnosis directly from blood.
Opota O, Jaton K, Greub G. Opota O, et al. Clin Microbiol Infect. 2015 Apr;21(4):323-31. doi: 10.1016/j.cmi.2015.02.005. Epub 2015 Feb 14. Clin Microbiol Infect. 2015. PMID: 25686695 Review. - Emerging commercial molecular tests for the diagnosis of bloodstream infection.
Mwaigwisya S, Assiri RA, O'Grady J. Mwaigwisya S, et al. Expert Rev Mol Diagn. 2015 May;15(5):681-92. doi: 10.1586/14737159.2015.1029459. Epub 2015 Apr 12. Expert Rev Mol Diagn. 2015. PMID: 25866124 Review.
Cited by
- A prospective multicenter evaluation of direct molecular detection of blood stream infection from a clinical perspective.
Nieman AE, Savelkoul PHM, Beishuizen A, Henrich B, Lamik B, MacKenzie CR, Kindgen-Milles D, Helmers A, Diaz C, Sakka SG, Schade RP. Nieman AE, et al. BMC Infect Dis. 2016 Jun 30;16:314. doi: 10.1186/s12879-016-1646-4. BMC Infect Dis. 2016. PMID: 27364885 Free PMC article. - 16S rDNA droplet digital PCR for monitoring bacterial DNAemia in bloodstream infections.
Ziegler I, Lindström S, Källgren M, Strålin K, Mölling P. Ziegler I, et al. PLoS One. 2019 Nov 13;14(11):e0224656. doi: 10.1371/journal.pone.0224656. eCollection 2019. PLoS One. 2019. PMID: 31721817 Free PMC article. - How small modifications in laboratory workflow of blood cultures can have a significant impact on time to results.
Van den Poel B, Klak A, Desmet S, Verhaegen J. Van den Poel B, et al. Eur J Clin Microbiol Infect Dis. 2018 Sep;37(9):1753-1760. doi: 10.1007/s10096-018-3309-4. Epub 2018 Jun 30. Eur J Clin Microbiol Infect Dis. 2018. PMID: 29961166 - Nucleic acid-based methods for early detection of sepsis.
Singh SP. Singh SP. Ann Card Anaesth. 2017 Jan-Mar;20(1):112-113. doi: 10.4103/0971-9784.197850. Ann Card Anaesth. 2017. PMID: 28074810 Free PMC article. No abstract available. - Calculated parenteral initial treatment of bacterial infections: Economic aspects of antibiotic treatment.
Wilke M, Hübner C, Kämmerer W. Wilke M, et al. GMS Infect Dis. 2020 Mar 26;8:Doc03. doi: 10.3205/id000047. eCollection 2020. GMS Infect Dis. 2020. PMID: 32373428 Free PMC article.
References
- O’Brien JM Jr, Ali NA, Aberegg SK, Abraham E. Sepsis. Am J Med. 2007;120: 1012–1022. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
EM holds a Miguel Servet position for researchers in the Spanish National Health System (MS09/00044, Instituto de Salud Carlos III, Ministerio de Economía y Competitividad). This work was partially funded by grant number PTA2011-5619-T (“Personal Técnico de Apoyo”) from “Ministerio de Ciencia e Innovación” (MICINN), “Plan Nacional de Investigación científica, Desarrollo e Innovación Tecnológica (I+D+I)” and the “Sociedad Española de Enfermedades Infecciosas y de Microbiología Clínica” (SEIMC) (EJL). All reagents and materials necessary for IRIDICA testing were supplied by Ibis Biosciences. This institution had no role in study design, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous