Mutations in TRAF3IP1/IFT54 reveal a new role for IFT proteins in microtubule stabilization - PubMed (original) (raw)
Anita Becker-Heck 3, Rebecca Ryan 1 2, Kristina Weber 4, Emilie Filhol 1 2, Pauline Krug 1 2, Jan Halbritter 5 6, Marion Delous 1 2, Marie-Christine Lasbennes 3, Bolan Linghu 7, Edward J Oakeley 3, Mohammed Zarhrate 2 8, Patrick Nitschké 2 9, Meriem Garfa-Traore 10, Fabrizio Serluca 7, Fan Yang 7, Tewis Bouwmeester 3, Lucile Pinson 11, Elisabeth Cassuto 12, Philippe Dubot 13, Neveen A Soliman Elshakhs 14, José A Sahel 15 16, Rémi Salomon 1 2 17, Iain A Drummond 18 19, Marie-Claire Gubler 1 2, Corinne Antignac 1 2 20, Salahdine Chibout 3, Joseph D Szustakowski 7, Friedhelm Hildebrandt 5, Esben Lorentzen 4, Andreas W Sailer 3, Alexandre Benmerah 1 2, Pierre Saint-Mezard 3, Sophie Saunier 1 2
Affiliations
- PMID: 26487268
- PMCID: PMC4617596
- DOI: 10.1038/ncomms9666
Mutations in TRAF3IP1/IFT54 reveal a new role for IFT proteins in microtubule stabilization
Albane A Bizet et al. Nat Commun. 2015.
Abstract
Ciliopathies are a large group of clinically and genetically heterogeneous disorders caused by defects in primary cilia. Here we identified mutations in TRAF3IP1 (TNF Receptor-Associated Factor Interacting Protein 1) in eight patients from five families with nephronophthisis (NPH) and retinal degeneration, two of the most common manifestations of ciliopathies. TRAF3IP1 encodes IFT54, a subunit of the IFT-B complex required for ciliogenesis. The identified mutations result in mild ciliary defects in patients but also reveal an unexpected role of IFT54 as a negative regulator of microtubule stability via MAP4 (microtubule-associated protein 4). Microtubule defects are associated with altered epithelialization/polarity in renal cells and with pronephric cysts and microphthalmia in zebrafish embryos. Our findings highlight the regulation of cytoplasmic microtubule dynamics as a role of the IFT54 protein beyond the cilium, contributing to the development of NPH-related ciliopathies.
Figures
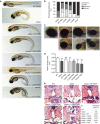
Figure 1. Identification of TRAF3IP1 mutations in patients with nephronophthisis and retinal degeneration.
Periodic acid schiff (a), trichrome (b) and silver methenamine (c) staining on kidney sections from individual NPH302-23 (a,b) and NPH1110-22 (c) revealed massive interstitial fibrosis (arrow heads) with cell infiltration, atrophic tubules with thickening of the basement membrane (arrows), as well as dilatation of proximal tubules (asterisks), characteristic of NPH. Scale bar, 50 μm. (d) Fundus photograph of individual NPH1110-22 showed characteristic aspects of RP, with pigmentary reorganization, papillary pallor and thin retinal vessels. (e) Left hand X-ray of individual NPH1110-22 showing short fingers (brachydactyly). (f) Organization of exons of TRAF3IP1 cDNA (top panel) and functional domains of IFT54 protein with an N-terminal calponin homology (CH) domain involved in tubulin binding, an Arginine-rich motif and a C-terminal coiled-coil domain involved in IFT20 binding. Black bars indicate positions of the identified mutations. Family numbers are underlined. H, homozygous; h, heterozygous.
Figure 2. Patients mutations do not rescue the ciliopathy-associated phenotypes characteristic of elipsa mutants.
(a) Lateral views of zebrafish larvae at 72 hpf of WT control, elipsa uninjected larvae and elipsa larvae injected with WT or mutant RNA constructs (p.R154* and p.V459R correspond to the human p.R155* and p.M520R mutations, respectively). Approximately 20% of V125M-injected elipsa larvae displayed an alternative stunted phenotype with pronephric cysts and eye defects, but lacking the characteristic body axis curvature. Scale bar, 0.5 mm. (b) Phenotype distribution as determined by quantification of angle of body axis curvature (_n_≥30, 4 independent experiments). (c) Eye phenotypes (5 dpf) of WT control, elipsa uninjected larvae and elipsa larvae injected with WT/mutant RNA constructs, lateral views, anterior to the left. Scale bar, 0.1 mm. (d) Surface area of the retina (mean ± s.d. of _n_=10, 2 independent experiments, *_P_=0.05, **P<0.01, and ***P<0.001, Dunnett's multiple-comparison test). (e) H&E staining of histological cross sections of elipsa mutant larvae injected with WT or mutant RNA constructs at 72 hpf. Gross cystic dilations of the glomerular region extending to the pronephric tubule are indicated by asterisks. Scale bar, 20 μm. (f) Percentage of pronephric cysts in elipsa mutant larvae as well as rescued larvae (_n_≥30, 4 independent experiments).
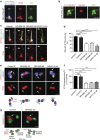
Figure 3. Mutations of TRAF3IP1 impair IFT54 ciliary trafficking.
(a) Fibroblasts transfected with GFP–IFT54-WT were fixed with MeOH and stained for GFP (green), IFT54 (red) and acetylated α-tubulin (blue, cilia). The base and the tip of the cilia are indicated by arrowhead and asterisk respectively. Scale bar, 1 μm. (b) _Traf3ip1_-KD mIMCD3 cells stably expressing GFP-tagged IFT54 WT were fixed with 4% PFA and stained for Cep164 (distal appendages, red). Scale bar, 1 μm. (c) Ciliary distribution of IFT54 in serum-starved control and patients' fibroblasts stained for IFT54 (red), acetylated α-tubulin (green, cilia) and the basal body marker γ-tubulin (blue). Scale bar, 1 μm. (d) Percentage of cilia with IFT54 at the distal tip of cilia (arrows in (c), mean ± s.e.m. of _n_=4 experiments (that is, ≈100 cilia), ***P<0.001, Bonferronni's multiple-comparison test). (e) Distribution of IFT54 at the basal body in ciliated fibroblasts stained for IFT54 (red) and for γ-tubulin (blue) and centrin (green), markers of proximal and distal parts of centrioles, respectively. A schematic representation of the orientation of the two centrioles, with the localization of the distal (DAP) and subdistal (sDAP) appendages is shown. Scale bar, 1 μm. (f) Intensity of IFT54 staining at the transition fibres/transition zone (TZ, arrows in (e), mean ± s.e.m. of _n_=3 experiments (that is, ≈50 cilia), ***P<0.001, Dunn's post-hoc test). (g) Fibroblasts from control or affected individuals were fixed and stained for ODF2 (red, subdistal appendages) and IFT54 (green) and analysed by STED microscopy. A schematic representation of the orientation of the analysed centrioles is shown. Arrows in g indicate the pool of IFT54 present at the distal tip of the mother centriole corresponding to the transition fibers/transition zone. Scale bars, 0.25 μm.
Figure 4. TRAF3IP1 mutations impair folding of the CH domain and interaction with MAP4.
(a) Crystal structure of the CH domain of _Mm_IFT54 (based on PDB entry 2EQO) showing that the I17 and V125 residues locate in conserved hydrophobic pockets (dotted line circles). The mutant residues S17, A125 and M125 were introduced (red) to show their effects on these hydrophobic pockets. (b) Lysates from HEK293T cells co-expressing Flag-tagged WT or mutant forms of _Mm_IFT54 (p.K155*, p.I453R and p.M458Mfs3* correspond to the human mutations p.R155*, p.M520R and p.M525Mfs3*) and GFP-MAP4 were immunoprecipitated with an anti-GFP antibody. The co-immunoprecipitation of GFP-MAP4 and Flag-IFT54 constructs was followed by western blot (WB) using GFP and Flag antibodies. (c) Serum-starved fibroblasts were fixed in PFA to visualize ciliary MAP4 (red; acetylated α-tubulin, green). Scale bar, 2 μm. (d) Intensity of ciliary MAP4 staining (mean ± s.e.m. of _n_=5 experiments (that is ≈150 cilia, *P<0.05, ***P<0.001, Dunn's post-hoc test).
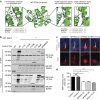
Figure 5. Mutations of TRAF3IP1 increase MAP4 expression, causing cytoplasmic microtubule stabilization.
(a) Expression of MAP4 and GAPDH in control and patients' fibroblasts were analysed by WB. (b) Relative expression of MAP4 normalized to that of GAPDH (mean ± s.e.m. of _n_=5 experiments, *P<0.05, Dunn's post-hoc test). (c) Fibroblasts were stained for acetylated α-tubulin (green). Scale bar, 10 μm. (d) Kidney biopsies from control and NPH638-21 and NPH302-23 affected individuals were stained for MAP4 (red), acetylated α-tubulin (green) and with peanut agglutinin (PNA, light blue, distal tubules). Scale bar, 25 μm. (e) Fibroblasts treated for 10 min on ice (to depolymerize the microtubules) were fixed with MeOH (to visualize MAP4 on microtubules) and stained for α-tubulin (green), γ-tubulin (light blue) and MAP4 (red). Scale bar, 10 μm. (f) Fibroblasts were stained for α-tubulin (green) and the microtubule plus-tip associated protein EB1 (red). Scale bar, 2 μm. (g) WT and elipsa embryos were injected with EB3-GFP to follow the dynamics of the growing ends of microtubules which were analysed by time lapse confocal microscopy and Imaris tracking software. Pseudo colours were used to visualize the speed of EB3 comets (from blue (slow) to red (fast)). (h) Track speed analysis of EB3-GFP comets in WT and elipsa embryos (_n_=6, mean ± s.d., ***P<0.001, _t-_test).
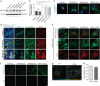
Figure 6. TRAF3IP1 mutations lead to epithelialization and polarity defects.
(a) mIMCD3 cells grown until confluence on filters were subjected to Ca2+-free medium to disrupt the tight junctions. Six hours after Ca2+ addition, cells were analysed by immunofluorescence using the apical marker Gp135 (red) and β-catenin (light blue) to stain the cell junctions. Scale bar, 10 μm. (b) Following Ca2+ switch, tight junction re-formation was assessed by measurement of trans-epithelial resistance (TER) at different time points (mean ± s.e.m. of _n_=5 independent experiments, two-way ANOVA; NS: not-significant, ***P<0.001 at 6 h). (c) Height of mIMCD3 cells grown on filters measured as the distance from the base to the top of the cells (GFP staining, not shown; mean ± s.d. of _n_≥20, from 3 independent experiments, ***P<0.001, Bonferonni's multiple-comparison test). (d) Expression of the apical marker Gp135 was analysed by Western blot with α-tubulin as a loading control. (e) mIMCD3 cells grown in matrigel 3D matrix to form spheroids were stained for ZO1 (tight junctions, red) and analysed by confocal microscopy. Arrows indicate ZO-1 at the apical junctions, while arrow heads point to mislocalized ZO-1. Equatorial sections of representative spheres are shown for each cell line. Scale bars, 10 μm. (f) Percentage of abnormal spheroids (no/small lumen filled with cells) (mean ± s.d., _n_=80 spheroids from 2 independent experiments, ***_P_≤0,001, **P<0.002, Bonferonni's multiple-comparison test).
Figure 7. Defects in TRAF3IP1 mutants are mediated by MAP4.
(a) Lateral views of WT zebrafish embryos injected with map4 morpholino at 48 hpf and phenotype distribution in WT embryos injected with control or map4 morpholino. (b) Lateral views of elipsa zebrafish embryos injected with map4 morpholino at 48 hpf and phenotype distribution in elipsa mutant embryos injected with control or map4 morpholino (data shown as combined result of _n_=3 independent experiments). Scale bars, 1 mm. (c) Relative expression of Map4 normalized to that of Hprt was analysed by qPCR in control and _Traf3ip1_-KD mIMCD3 cells stably expressing GFP or GFP-IFT54 mutants and Map4 shRNA. (d) Control and _Traf3ip1_-KD/ _Map4_-KD mIMCD3 cells expressing either GFP or IFT54-GFP fusions were fixed in MeOH and stained for acetylated α-tubulin (red) and γ-tubulin (light blue). Scale bar, 10 μm. (e) Six hours after Ca2+ switch, mIMCD3 cells grown until confluence on filters were fixed with 4% PFA and stained for the apical marker Gp135 (red). Scale bar, 10 μm. (f) Percentage of normal spheroids of control and _Traf3ip1_-KD/ _Map4_-KD mIMCD3 cells expressing either GFP or IFT54-GFP fusions grown on Matrigel for 5 days (mean ± s.d., _n_≥100 spheroids from 3 independent experiments, ***_P_≤0,0001, *P<0.012, Bonferonni's multiple-comparison test).
Similar articles
- Retinal Degeneration Animal Models in Bardet-Biedl Syndrome and Related Ciliopathies.
Delvallée C, Dollfus H. Delvallée C, et al. Cold Spring Harb Perspect Med. 2023 Jan 3;13(1):a041303. doi: 10.1101/cshperspect.a041303. Cold Spring Harb Perspect Med. 2023. PMID: 36596648 Free PMC article. Review. - Ccrk-Mak/Ick signaling is a ciliary transport regulator essential for retinal photoreceptor survival.
Chaya T, Maeda Y, Tsutsumi R, Ando M, Ma Y, Kajimura N, Tanaka T, Furukawa T. Chaya T, et al. Life Sci Alliance. 2024 Sep 18;7(11):e202402880. doi: 10.26508/lsa.202402880. Print 2024 Nov. Life Sci Alliance. 2024. PMID: 39293864 Free PMC article. - Ciliary length regulation by intraflagellar transport in zebrafish.
Sun Y, Chen Z, Jin M, Xie H, Zhao C. Sun Y, et al. Elife. 2024 Dec 13;13:RP93168. doi: 10.7554/eLife.93168. Elife. 2024. PMID: 39671305 Free PMC article. - Can a Liquid Biopsy Detect Circulating Tumor DNA With Low-passage Whole-genome Sequencing in Patients With a Sarcoma? A Pilot Evaluation.
Anderson CJ, Yang H, Parsons J, Ahrens WA, Jagosky MH, Hsu JH, Patt JC, Kneisl JS, Steuerwald NM. Anderson CJ, et al. Clin Orthop Relat Res. 2025 Jan 1;483(1):39-48. doi: 10.1097/CORR.0000000000003161. Epub 2024 Jun 21. Clin Orthop Relat Res. 2025. PMID: 38905450 - Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
Cited by
- Ciliopathy-associated IQCB1/NPHP5 protein is required for mouse photoreceptor outer segment formation.
Ronquillo CC, Hanke-Gogokhia C, Revelo MP, Frederick JM, Jiang L, Baehr W. Ronquillo CC, et al. FASEB J. 2016 Oct;30(10):3400-3412. doi: 10.1096/fj.201600511R. Epub 2016 Jun 21. FASEB J. 2016. PMID: 27328943 Free PMC article. - CRISPR/Cas9-mediated Genomic Editing of Cluap1/IFT38 Reveals a New Role in Actin Arrangement.
Beyer T, Bolz S, Junger K, Horn N, Moniruzzaman M, Wissinger Y, Ueffing M, Boldt K. Beyer T, et al. Mol Cell Proteomics. 2018 Jul;17(7):1285-1294. doi: 10.1074/mcp.RA117.000487. Epub 2018 Apr 3. Mol Cell Proteomics. 2018. PMID: 29615496 Free PMC article. - Agonists of prostaglandin E2 receptors as potential first in class treatment for nephronophthisis and related ciliopathies.
Garcia H, Serafin AS, Silbermann F, Porée E, Viau A, Mahaut C, Billot K, Birgy É, Garfa-Traore M, Roy S, Ceccarelli S, Mehraz M, Rodriguez PC, Deleglise B, Furio L, Jabot-Hanin F, Cagnard N, Del Nery E, Fila M, Sin-Monnot S, Antignac C, Lyonnet S, Krug P, Salomon R, Annereau JP, Benmerah A, Delous M, Briseño-Roa L, Saunier S. Garcia H, et al. Proc Natl Acad Sci U S A. 2022 May 3;119(18):e2115960119. doi: 10.1073/pnas.2115960119. Epub 2022 Apr 28. Proc Natl Acad Sci U S A. 2022. PMID: 35482924 Free PMC article. - MIP-T3 Expression Associated with Defects of Ciliogenesis in Airway of COPD Patients.
Wang WJ, Yang SF, Gao ZR, Luo ZR, Liu YL, Gao XL. Wang WJ, et al. Can Respir J. 2020 Feb 10;2020:1350872. doi: 10.1155/2020/1350872. eCollection 2020. Can Respir J. 2020. PMID: 32104517 Free PMC article. - Chlamydomonas IFT25 is dispensable for flagellar assembly but required to export the BBSome from flagella.
Dong B, Wu S, Wang J, Liu YX, Peng Z, Meng DM, Huang K, Wu M, Fan ZC. Dong B, et al. Biol Open. 2017 Nov 15;6(11):1680-1691. doi: 10.1242/bio.026278. Biol Open. 2017. PMID: 28838966 Free PMC article.
References
- Tobin J. L. & Beales P. L. The nonmotile ciliopathies. Genet. Med. 11, 386–402 (2009) . - PubMed
- Ishikawa H. & Marshall W. F. Ciliogenesis: building the cell's antenna. Nat. Rev. Mol. Cell. Biol. 12, 222–234 (2011) . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK053093/DK/NIDDK NIH HHS/United States
- R01 DK068306/DK/NIDDK NIH HHS/United States
- DK068306/DK/NIDDK NIH HHS/United States
- Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous