Genomic analysis of germ line and somatic variants in familial myelodysplasia/acute myeloid leukemia - PubMed (original) (raw)
Clinical Trial
. 2015 Nov 26;126(22):2484-90.
doi: 10.1182/blood-2015-04-641100. Epub 2015 Oct 22.
Khateriaa Pyrtel 2, Krishna-Latha Kanchi 3, Jin Shao 2, Daniel Koboldt 4, Christopher A Miller 3, Dong Shen 5, Robert Fulton 3, Michelle O'Laughlin 3, Catrina Fronick 3, Iskra Pusic 6, Geoffrey L Uy 6, Evan M Braunstein 7, Mark Levis 7, Julie Ross 8, Kevin Elliott 2, Sharon Heath 2, Allan Jiang 6, Peter Westervelt 9, John F DiPersio 9, Daniel C Link 9, Matthew J Walter 9, John Welch 9, Richard Wilson 10, Timothy J Ley 4, Lucy A Godley 1, Timothy A Graubert 11
Affiliations
- PMID: 26492932
- PMCID: PMC4661171
- DOI: 10.1182/blood-2015-04-641100
Clinical Trial
Genomic analysis of germ line and somatic variants in familial myelodysplasia/acute myeloid leukemia
Jane E Churpek et al. Blood. 2015.
Abstract
Familial clustering of myelodysplastic syndromes (MDSs) and acute myeloid leukemia (AML) can be caused by inherited factors. We screened 59 individuals from 17 families with 2 or more biological relatives with MDS/AML for variants in 12 genes with established roles in predisposition to MDS/AML, and identified a pathogenic germ line variant in 5 families (29%). Extending the screen with a panel of 264 genes that are recurrently mutated in de novo AML, we identified rare, nonsynonymous germ line variants in 4 genes, each segregating with MDS/AML in 2 families. Somatic mutations are required for progression to MDS/AML in these familial cases. Using a combination of targeted and exome sequencing of tumor and matched normal samples from 26 familial MDS/AML cases and asymptomatic carriers, we identified recurrent frameshift mutations in the cohesin-associated factor PDS5B, co-occurrence of somatic ASXL1 mutations with germ line GATA2 mutations, and recurrent mutations in other known MDS/AML drivers. Mutations in genes that are recurrently mutated in de novo AML were underrepresented in the familial MDS/AML cases, although the total number of somatic mutations per exome was the same. Lastly, clonal skewing of hematopoiesis was detected in 67% of young, asymptomatic RUNX1 carriers, providing a potential biomarker that could be used for surveillance in these high-risk families.
© 2015 by The American Society of Hematology.
Figures
Figure 1
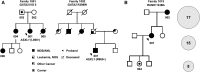
Familial MDS/AML pedigrees. (A) Partial pedigrees of families 1001 and 1002 with _GATA2_-associated MDS/AML; complete pedigrees are provided in supplemental Figure 1. Subjects who provided samples for sequencing are indicated by numerals. GATA2 genotypes are provided in Table 1. Two individuals with MDS acquired somatic ASXL1 mutations, as shown. (B) Partial pedigree of family 1015 with _RUNX1_-associated MDS/AML (left); complete pedigree is provided in supplemental Figure 1. The number of somatic variants (SNVs, indels) detected by exome sequencing in the 3 individuals (indicated by numerals in the pedigree) is shown in the circles (right). The size of the circles is proportional to the median VAFs of somatic SNVs in each case. NOS, not otherwise specified.
Figure 2
Somatic variants in familial MDS/AML vs de novo AML. (A) Targeted sequencing of known RMGs demonstrated fewer mutations in familial cases compared to de novo cases (median, 2.0 vs 5.0; P = .0013 by 2-tailed Mann-Whitney). (B) Somatic mutation VAFs detected by exome sequencing in asymptomatic RUNX1 carriers and familial MDS and AML cases from GATA2 or RUNX1 families are shown. Age at sample collection is indicated by the x-axis labels (black, RUNX1 carrier; red, GATA2 carrier). Clonal hematopoiesis was detectable in 6 of 9 asymptomatic RUNX1 carriers.
Figure 3
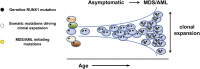
Clonal evolution in RUNX1 carriers. Asymptomatic carriers of pathogenic germ line RUNX1 variants develop early-onset clonal hematopoiesis (cumulative risk of 81% by age 50 years). As depicted in the model, this finding provides a rationale for testing the hypothesis that clonal hematopoiesis may provide a biomarker for early detection of disease progression in high-risk families.
Similar articles
- Genetic predisposition to myelodysplastic syndrome and acute myeloid leukemia in children and young adults.
Babushok DV, Bessler M, Olson TS. Babushok DV, et al. Leuk Lymphoma. 2016;57(3):520-36. doi: 10.3109/10428194.2015.1115041. Epub 2015 Dec 23. Leuk Lymphoma. 2016. PMID: 26693794 Free PMC article. - Familial myelodysplasia and acute myeloid leukaemia--a review.
Owen C, Barnett M, Fitzgibbon J. Owen C, et al. Br J Haematol. 2008 Jan;140(2):123-32. doi: 10.1111/j.1365-2141.2007.06909.x. Br J Haematol. 2008. PMID: 18173751 Review. - Mutational landscape of patients with acute myeloid leukemia or myelodysplastic syndromes in the context of RUNX1 mutation.
Wang K, Zhou F, Cai X, Chao H, Zhang R, Chen S. Wang K, et al. Hematology. 2020 Dec;25(1):211-218. doi: 10.1080/16078454.2020.1765561. Hematology. 2020. PMID: 32476595 - Aplastic anemia and clonal evolution: germ line and somatic genetics.
Shimamura A. Shimamura A. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;2016(1):74-82. doi: 10.1182/asheducation-2016.1.74. Hematology Am Soc Hematol Educ Program. 2016. PMID: 27913465 Free PMC article. Review. - Germ-line GATA2 p.THR354MET mutation in familial myelodysplastic syndrome with acquired monosomy 7 and ASXL1 mutation demonstrating rapid onset and poor survival.
Bödör C, Renneville A, Smith M, Charazac A, Iqbal S, Etancelin P, Cavenagh J, Barnett MJ, Kramarzová K, Krishnan B, Matolcsy A, Preudhomme C, Fitzgibbon J, Owen C. Bödör C, et al. Haematologica. 2012 Jun;97(6):890-4. doi: 10.3324/haematol.2011.054361. Epub 2012 Jan 22. Haematologica. 2012. PMID: 22271902 Free PMC article.
Cited by
- Review of guidelines for the identification and clinical care of patients with genetic predisposition for hematological malignancies.
Schlegelberger B, Mecucci C, Wlodarski M. Schlegelberger B, et al. Fam Cancer. 2021 Oct;20(4):295-303. doi: 10.1007/s10689-021-00263-z. Epub 2021 May 31. Fam Cancer. 2021. PMID: 34057692 Free PMC article. Review. - Clonal hematopoiesis in the inherited bone marrow failure syndromes.
Tsai FD, Lindsley RC. Tsai FD, et al. Blood. 2020 Oct 1;136(14):1615-1622. doi: 10.1182/blood.2019000990. Blood. 2020. PMID: 32736377 Free PMC article. Review. - Genetic predisposition to MDS: clinical features and clonal evolution.
Kennedy AL, Shimamura A. Kennedy AL, et al. Blood. 2019 Mar 7;133(10):1071-1085. doi: 10.1182/blood-2018-10-844662. Epub 2019 Jan 22. Blood. 2019. PMID: 30670445 Free PMC article. Review. - Runx1-R188Q germ line mutation induces inflammation and predisposition to hematologic malignancies in mice.
Ahmad MH, Hegde M, Wong WJ, Mohammadhosseini M, Garrett L, Carrascoso A, Issac N, Ebert B, Silva JC, Pihan G, Zhu LJ, Wolfe SA, Agarwal A, Liu PP, Castilla LH. Ahmad MH, et al. Blood Adv. 2023 Dec 12;7(23):7304-7318. doi: 10.1182/bloodadvances.2023010398. Blood Adv. 2023. PMID: 37756546 Free PMC article. - Germline mutations: many roles in leukemogenesis.
Chen KZ, Kazi R, Porter CC, Qu CK. Chen KZ, et al. Curr Opin Hematol. 2020 Jul;27(4):288-293. doi: 10.1097/MOH.0000000000000596. Curr Opin Hematol. 2020. PMID: 32487806 Free PMC article. Review.
References
- Song WJ, Sullivan MG, Legare RD, et al. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nat Genet. 1999;23(2):166–175. - PubMed
- National Center for Biotechnology Information. OMIM: online Mendelian inheritance in man. http://www.ncbi.nlm.nih.gov/omim, article #601399. Accessed April 15, 2015.
- Smith ML, Cavenagh JD, Lister TA, Fitzgibbon J. Mutation of CEBPA in familial acute myeloid leukemia. N Engl J Med. 2004;351(23):2403–2407. - PubMed
- Sellick GS, Spendlove HE, Catovsky D, Pritchard-Jones K, Houlston RS. Further evidence that germline CEBPA mutations cause dominant inheritance of acute myeloid leukaemia. Leukemia. 2005;19(7):1276–1278. - PubMed
- National Center for Biotechnology Information. OMIM: online Mendelian inheritance in man. http://www.ncbi.nlm.nih.gov/omim, article #116897. Accessed April 15, 2015.
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000430/TR/NCATS NIH HHS/United States
- K12 CA139160/CA/NCI NIH HHS/United States
- T32 HL007525/HL/NHLBI NIH HHS/United States
- K05 CA157439/CA/NCI NIH HHS/United States
- CA157439/CA/NCI NIH HHS/United States
- K12 HL087169/HL/NHLBI NIH HHS/United States
- CA101937/CA/NCI NIH HHS/United States
- P01 CA101937/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous