Tau positron emission tomographic imaging in aging and early Alzheimer disease - PubMed (original) (raw)
doi: 10.1002/ana.24546. Epub 2015 Dec 15.
Keith A Johnson 1 2 3 4 5, Aaron Schultz 1 4 6, J Alex Becker 1 3, Jorge Sepulcre 1 3 5 6, Dorene Rentz 2 4 5, Elizabeth Mormino 2 4, Jasmeer Chhatwal 2 4 5, Rebecca Amariglio 2 4 5, Kate Papp 2 4 5, Gad Marshall 2 4 5, Mark Albers 2 5, Samantha Mauro 1 3, Lesley Pepin 1 3, Jonathan Alverio 1 3, Kelly Judge 1 3, Marlie Philiossaint 1 3, Timothy Shoup 1 3, Daniel Yokell 1 3 5, Bradford Dickerson 1 2 5 6, Teresa Gomez-Isla 2 5, Bradley Hyman 2 5, Neil Vasdev 1 3 5, Reisa Sperling 2 4 5 6
Affiliations
- PMID: 26505746
- PMCID: PMC4738026
- DOI: 10.1002/ana.24546
Tau positron emission tomographic imaging in aging and early Alzheimer disease
Keith A Johnson et al. Ann Neurol. 2016 Jan.
Abstract
Objective: Detection of focal brain tau deposition during life could greatly facilitate accurate diagnosis of Alzheimer disease (AD), staging and monitoring of disease progression, and development of disease-modifying therapies.
Methods: We acquired tau positron emission tomography (PET) using (18)F T807 (AV1451), and amyloid-β PET using (11)C Pittsburgh compound B (PiB) in older clinically normal individuals, and symptomatic patients with mild cognitive impairment or mild AD dementia.
Results: We found abnormally high cortical (18)F T807 binding in patients with mild cognitive impairment and AD dementia compared to clinically normal controls. Consistent with the neuropathology literature, the presence of elevated neocortical (18)F T807 binding particularly in the inferior temporal gyrus was associated with clinical impairment. The association of cognitive impairment was stronger with inferior temporal (18)F T807 than with mean cortical (11)C PIB. Regional (18)F T807 was correlated with mean cortical (11)C PiB among both impaired and control subjects.
Interpretation: These findings suggest that (18)F T807 PET could have value as a biomarker that reflects both the progression of AD tauopathy and the emergence of clinical impairment.
© 2015 American Neurological Association.
Conflict of interest statement
Potential Conflict of Interests: AS, RB, JAB, JS, DR, EM, JC, RA, KP, GM, SM, LP, JA, KJ, MP, TS, DY, and TG have no competing interests.
Figures
Figure 1
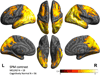
Cortical patterns of 18F T807 binding. Coronal 18F T807 PET images (top row) and whole-brain surface renderings of SUVR (second row) from 3 normal (CN) and 4 impaired (2 MCI and 2 AD dementia) participants. Top row A. A 71 year-old CN with low Aβ by PiB PET (DVR=1.0) had low, non-specific 18F T807 binding in cortex, consistent with a Braak Stage less than III/IV. B. A 74 year-old CN with high Aβ (DVR=1.2) with 18F T807 binding in inferior temporal cortex, left>right, consistent with Braak Stage III/IV. C. A 79 year-old CN, high Aβ (DVR=1.8) had binding in inferior temporal neocortex, consistent with Braak Stage of III/IV. B and C show focally intense subcortical uptake that is likely due to off-target binding (see Discussion). D–G. Cognitively impaired participants all with high Aβ and with successively greater levels of cortical 18F T807 binding successively involving temporal, parietal, frontal and occipital cortices. Second row: 18F T807 SUVR calculated at vertices (see Methods) indicating the extent of cortical binding, with left hemisphere views (lateral, inferior, superior, medial) at left. The 52 year-old AD dementia patient (G) showed confluent 18F T807 binding that is nearly pancortical, sparing only portions of primary cortex and consistent with Braak Stage V/VI. MMSE= mini-mental state exam PiB= Pittsburgh Compound B DVR= distribution volume ratio, mean cortical SUVR= standardized uptake value, cerebellar reference Dx= classification (CN, clinically normal; MCI, mild cognitive impairment; AD mild AD dementia) PET Braak= Estimate of Braak Stages is based on the anatomic pattern of T807 binding assessed visually and quantitatively in regions and full volume data.
Figure 2
Cortical distribution of T807 binding, contrast between the combined MCI/AD group (N=19) and the CN group (N=56; threshold p< 10^−5).
Figure 3
Regional T807 binding according to diagnostic group. T807 standardized uptake value radio (SUVR) in regions-of-interest (ROI) by diagnostic group (CN, circles; MCI, triangles; AD, squares) and amyloid status (high Aβ, red, = PiB DVR 1.2 or greater), showing range, mean, interquartile range. t-test: *p<0.01, **p<0.001
Figure 4
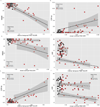
A – F. Correlations of Tau pathology measured with 18F T807 and Aβ pathology measured with 11C PiB with Mini-Mental State Examination, CDR Sum of Boxes, and Logical Memory 2. Clinically normal subjects are represented with circles, MCI with triangles, and AD with squares; red indicates high Aβ (PiB DVR >1.2) and black low Aβ (PiB DVR ≤1.2). Spearman correlations (rho) are given below for each PET measure versus MMSE, CDR SB, or LM. A. MMSE vs. Inferior temporal T807 [Table: see text] B. MMSE vs. mean cortical PiB DVR [Table: see text] C. CDR SB vs. Inferior temporal T807 [Table: see text] D. CDR SB vs. mean cortical PiB DVR [Table: see text] E. LM vs. Inferior temporal T807 [Table: see text] F. LM vs. mean cortical PiB DVR [Table: see text]
Figure 5
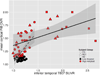
Correlations of tau pathology measured with inferior temporal 18F T807 and Aβ pathology measured with mean cortical 11C PiB. Clinically normal subjects are represented with circles, MCI with triangles, and AD with squares; red indicates high Aβ (PiB DVR >1.2) and black low Aβ (PiB DVR ≤1.2). Separate linear fit lines (dashed for CN, solid for MCI/AD) are shown to aid inspection. ρ = Spearman’s Rho. [Table: see text]
Similar articles
- Region-Specific Association of Subjective Cognitive Decline With Tauopathy Independent of Global β-Amyloid Burden.
Buckley RF, Hanseeuw B, Schultz AP, Vannini P, Aghjayan SL, Properzi MJ, Jackson JD, Mormino EC, Rentz DM, Sperling RA, Johnson KA, Amariglio RE. Buckley RF, et al. JAMA Neurol. 2017 Dec 1;74(12):1455-1463. doi: 10.1001/jamaneurol.2017.2216. JAMA Neurol. 2017. PMID: 28973551 Free PMC article. - Tau Positron Emission Tomographic Imaging in the Lewy Body Diseases.
Gomperts SN, Locascio JJ, Makaretz SJ, Schultz A, Caso C, Vasdev N, Sperling R, Growdon JH, Dickerson BC, Johnson K. Gomperts SN, et al. JAMA Neurol. 2016 Nov 1;73(11):1334-1341. doi: 10.1001/jamaneurol.2016.3338. JAMA Neurol. 2016. PMID: 27654968 Free PMC article. - Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease.
Ossenkoppele R, Schonhaut DR, Schöll M, Lockhart SN, Ayakta N, Baker SL, O'Neil JP, Janabi M, Lazaris A, Cantwell A, Vogel J, Santos M, Miller ZA, Bettcher BM, Vossel KA, Kramer JH, Gorno-Tempini ML, Miller BL, Jagust WJ, Rabinovici GD. Ossenkoppele R, et al. Brain. 2016 May;139(Pt 5):1551-67. doi: 10.1093/brain/aww027. Epub 2016 Mar 8. Brain. 2016. PMID: 26962052 Free PMC article. - In vivo tau PET imaging in dementia: Pathophysiology, radiotracer quantification, and a systematic review of clinical findings.
Hall B, Mak E, Cervenka S, Aigbirhio FI, Rowe JB, O'Brien JT. Hall B, et al. Ageing Res Rev. 2017 Jul;36:50-63. doi: 10.1016/j.arr.2017.03.002. Epub 2017 Mar 15. Ageing Res Rev. 2017. PMID: 28315409 Review. - Applications of amyloid, tau, and neuroinflammation PET imaging to Alzheimer's disease and mild cognitive impairment.
Chandra A, Valkimadi PE, Pagano G, Cousins O, Dervenoulas G, Politis M; Alzheimer's Disease Neuroimaging Initiative. Chandra A, et al. Hum Brain Mapp. 2019 Dec 15;40(18):5424-5442. doi: 10.1002/hbm.24782. Epub 2019 Sep 14. Hum Brain Mapp. 2019. PMID: 31520513 Free PMC article. Review.
Cited by
- The impact of tau-PET in a selected memory clinic cohort: rationale and design of the TAP-TAU study.
Vermeiren MR, Somsen J, Luurtsema G, Reesink FE, Verwey NA, Hempenius L, Tolboom N, Biessels GJ, Biesbroek JM, Vernooij MW, Veldhuijzen van Zanten SEM, Seelaar H, Coomans EM, Teunissen CE, Lemstra AW, van Harten AC, Visser LNC, van der Flier WM, van de Giessen E, Ossenkoppele R. Vermeiren MR, et al. Alzheimers Res Ther. 2024 Oct 19;16(1):230. doi: 10.1186/s13195-024-01588-4. Alzheimers Res Ther. 2024. PMID: 39427210 Free PMC article. - Biological mechanisms of resilience to tau pathology in Alzheimer's disease.
Svenningsson AL, Bocancea DI, Stomrud E, van Loenhoud A, Barkhof F, Mattsson-Carlgren N, Palmqvist S, Hansson O, Ossenkoppele R. Svenningsson AL, et al. Alzheimers Res Ther. 2024 Oct 12;16(1):221. doi: 10.1186/s13195-024-01591-9. Alzheimers Res Ther. 2024. PMID: 39396028 Free PMC article. - Amyloid-β deposition in basal frontotemporal cortex is associated with selective disruption of temporal mnemonic discrimination.
Vanderlip CR, Taylor L, Kim S, Harris AL, Tuteja N, Meza N, Escalante YY, McMillan L, Yassa MA, Adams JN. Vanderlip CR, et al. bioRxiv [Preprint]. 2024 Aug 26:2024.08.23.609449. doi: 10.1101/2024.08.23.609449. bioRxiv. 2024. PMID: 39253484 Free PMC article. Preprint. - Rationale and design of the BeyeOMARKER study: prospective evaluation of blood- and eye-based biomarkers for early detection of Alzheimer's disease pathology in the eye clinic.
Bader I, Groot C, Tan HS, Milongo JA, Haan JD, Verberk IMW, Yong K, Orellina J, Campbell S, Wilson D, van Harten AC, Kok PHB, van der Flier WM, Pijnenburg YAL, Barkhof F, van de Giessen E, Teunissen CE, Bouwman FH, Ossenkoppele R. Bader I, et al. Alzheimers Res Ther. 2024 Aug 21;16(1):190. doi: 10.1186/s13195-024-01545-1. Alzheimers Res Ther. 2024. PMID: 39169442 Free PMC article. - Blood tests could soon predict your risk of Alzheimer's.
Abbott A. Abbott A. Nature. 2024 Aug;632(8024):243-245. doi: 10.1038/d41586-024-02535-x. Nature. 2024. PMID: 39112619 No abstract available.
References
- Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. - PubMed
- Clark CM, Pontecorvo MJ, Beach TG, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 2012;11(8):669–678. - PubMed
- Rinne JO, Wong DF, Wolk DA, et al. [(18)F]Flutemetamol PET imaging and cortical biopsy histopathology for fibrillar amyloid β detection in living subjects with normal pressure hydrocephalus: pooled analysis of four studies. Acta Neuropathol. 2012;124(6):833–845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG037497/AG/NIA NIH HHS/United States
- R01 AG027435/AG/NIA NIH HHS/United States
- M01 RR002635/RR/NCRR NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
- P50 AG00513421/AG/NIA NIH HHS/United States
- U01 AG010483/AG/NIA NIH HHS/United States
- K23 AG049087/AG/NIA NIH HHS/United States
- R01 AG046396/AG/NIA NIH HHS/United States
- P01 AG036694/AG/NIA NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- U19 AG010483/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical