Plasma Membrane Repair: A Central Process for Maintaining Cellular Homeostasis - PubMed (original) (raw)
Review
Plasma Membrane Repair: A Central Process for Maintaining Cellular Homeostasis
Alisa D Blazek et al. Physiology (Bethesda). 2015 Nov.
Abstract
Plasma membrane repair is a conserved cellular response mediating active resealing of membrane disruptions to maintain homeostasis and prevent cell death and progression of multiple diseases. Cell membrane repair repurposes mechanisms from various cellular functions, including vesicle trafficking, exocytosis, and endocytosis, to mend the broken membrane. Recent studies increased our understanding of membrane repair by establishing the molecular machinery contributing to membrane resealing. Here, we review some of the key proteins linked to cell membrane repair.
©2015 Int. Union Physiol. Sci./Am. Physiol. Soc.
Conflict of interest statement
Noah Weisleder is Founder and Chief Scientific Officer of TRIM-edicine, a biotechnology company developing products targeting membrane repair, including rhMG53.
Figures
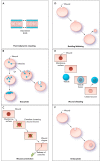
FIGURE 1.
Models of the plasma membrane repair process A: thermodynamic resealing occurs spontaneously due to tension produced by the disordered arrangement of the membrane phospholipids at the open edge of the break. This process is the most likely route of resealing for membrane breaks of ≤1 μm in diameter. B: exocytosis can contribute by trafficking intracellular vesicles to the wounded area where they can fuse with each other and the injured membrane to form a repair patch. C: wound constriction is mediated by caveolae. During this process, caveolae cluster and fuse around larger wounds, leading to wound constriction and intracellular fusion of caveolar endosomes. D: budding/blebbing of the membrane portion containing the wound site with release of the newly formed vesicles into the extracellular space also involves exocytosis. E: exocytosis of an intracellular patch and fusion to the wound site could result in the extracellular release or “shedding” of the wound site. F: endocytosis of wounds occurs via invagination of caveolar vesicles and subsequent intracellular fusion of caveolae.
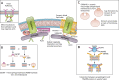
FIGURE 2.
Major membrane repair proteins and their hypothesized roles in the repair process Dysferlin's interaction with AHNAK is regulated by calpain, and cleavage of dysferlin can result in additional subunits that function in repair. Calpain may also regulate cytoskeletal structure and sarcomere remodeling. AHNAK may aid in cytoskeletal remodeling. MG53/TRIM72 and dysferlin form a vesicle lattice to close the wound. A: affixin, which also binds dysferlin, localizes to focal adhesions and may organize actin. B: ESCRT and acid sphingomyelinase (ASM) facilitate exocytosis and endocytosis. ESCRT has been found to be involved in both endocytosis and budding. ESCRT III can be recruited to the membrane, followed by blebbing of the membrane and shedding of the wound. ASM is secreted and cleaves sphingomyelin to generate ceramide, leading to membrane invagination of the injury site. C: the annexin and S100A10 complex binds dysferlin and may recruit AHNAK to the membrane due to annexin's ability to bind lipid rafts. Annexin/S100A10 may also bridge adjacent phospholipids to form endosomes. Annexin accumulates at the neck of membrane blebs to mediate microvesicle release. Annexin A6 may also “cap” the membrane repair patch. D: synaptotagmin and SNARE proteins interact at the plasma membrane via a conformational change in synaptotagmin present on synaptic vesicles to fuse the vesicles with the membrane.
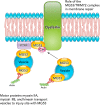
FIGURE 3.
Proposed roles of MG53/TRIM72 in mediating membrane repair MG53/TRIM72 interacts with phosphatidylserine in the plasma membrane in a complex containing dysferlin and Cav-3. MG53/TRIM72 and dysferlin close the membrane wound with vesicles. Vesicle transport is facilitated by myosin motor proteins. Cav-3 may regulate MG53/TRIM72-mediated membrane fusion and is enriched in caveolae or plasma membrane invaginations. PTRF may aid in the formation and stabilization of these caveolae by interaction with Cav-3 and MG53/TRIM72 through cholesterol. PTRF also binds and may help localize dysferlin.
Similar articles
- Membrane Tension Regulation is Required for Wound Repair.
Raj N, Weiß MS, Vos BE, Weischer S, Brinkmann F, Betz T, Trappmann B, Gerke V. Raj N, et al. Adv Sci (Weinh). 2024 Dec;11(48):e2402317. doi: 10.1002/advs.202402317. Epub 2024 Oct 3. Adv Sci (Weinh). 2024. PMID: 39360573 Free PMC article. - Calcium signaling in membrane repair.
Cheng X, Zhang X, Yu L, Xu H. Cheng X, et al. Semin Cell Dev Biol. 2015 Sep;45:24-31. doi: 10.1016/j.semcdb.2015.10.031. Epub 2015 Oct 27. Semin Cell Dev Biol. 2015. PMID: 26519113 Free PMC article. Review. - Approaches for plasma membrane wounding and assessment of lysosome-mediated repair responses.
Corrotte M, Castro-Gomes T, Koushik AB, Andrews NW. Corrotte M, et al. Methods Cell Biol. 2015;126:139-58. doi: 10.1016/bs.mcb.2014.11.009. Epub 2015 Jan 14. Methods Cell Biol. 2015. PMID: 25665445 Free PMC article. - Emerging role of the scaffolding protein Dlg1 in vesicle trafficking.
Walch L. Walch L. Traffic. 2013 Sep;14(9):964-73. doi: 10.1111/tra.12089. Traffic. 2013. PMID: 23829493 Review. - Defective membrane repair in dysferlin-deficient muscular dystrophy.
Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Bansal D, et al. Nature. 2003 May 8;423(6936):168-72. doi: 10.1038/nature01573. Nature. 2003. PMID: 12736685
Cited by
- Platelet Membrane: An Outstanding Factor in Cancer Metastasis.
Durán-Saenz NZ, Serrano-Puente A, Gallegos-Flores PI, Mendoza-Almanza BD, Esparza-Ibarra EL, Godina-González S, González-Curiel IE, Ayala-Luján JL, Hernández-Barrales M, Cueto-Villalobos CF, Frausto-Fierros SY, Burciaga-Hernandez LA, Mendoza-Almanza G. Durán-Saenz NZ, et al. Membranes (Basel). 2022 Feb 3;12(2):182. doi: 10.3390/membranes12020182. Membranes (Basel). 2022. PMID: 35207103 Free PMC article. Review. - Gut homeostasis, injury, and healing: New therapeutic targets.
Oncel S, Basson MD. Oncel S, et al. World J Gastroenterol. 2022 May 7;28(17):1725-1750. doi: 10.3748/wjg.v28.i17.1725. World J Gastroenterol. 2022. PMID: 35633906 Free PMC article. Review. - MG53 is dispensable for T-tubule maturation but critical for maintaining T-tubule integrity following cardiac stress.
Zhang C, Chen B, Wang Y, Guo A, Tang Y, Khataei T, Shi Y, Kutschke WJ, Zimmerman K, Weiss RM, Liu J, Benson CJ, Hong J, Ma J, Song LS. Zhang C, et al. J Mol Cell Cardiol. 2017 Nov;112:123-130. doi: 10.1016/j.yjmcc.2017.08.007. Epub 2017 Aug 16. J Mol Cell Cardiol. 2017. PMID: 28822805 Free PMC article. - Treatment with Recombinant Human MG53 Protein Increases Membrane Integrity in a Mouse Model of Limb Girdle Muscular Dystrophy 2B.
Gushchina LV, Bhattacharya S, McElhanon KE, Choi JH, Manring H, Beck EX, Alloush J, Weisleder N. Gushchina LV, et al. Mol Ther. 2017 Oct 4;25(10):2360-2371. doi: 10.1016/j.ymthe.2017.06.025. Epub 2017 Jul 3. Mol Ther. 2017. PMID: 28750735 Free PMC article. - Enhancing Membrane Repair Using Recombinant MG53/TRIM72 (rhMG53) Reduces Neurotoxicity in Alzheimer's Disease Models.
Bulgart HR, Lopez Perez MA, Weisleder N. Bulgart HR, et al. Biomolecules. 2025 Mar 15;15(3):418. doi: 10.3390/biom15030418. Biomolecules. 2025. PMID: 40149954 Free PMC article.
References
- Ampong BN, Imamura M, Matsumiya T, Yoshida M, Takeda S. Intracellular localization of dysferlin and its association with the dihydropyridine receptor. Acta Myol 24: 134–144, 2005. - PubMed
- Anderson LV, Davison K, Moss JA, Young C, Cullen MJ, Walsh J, Johnson MA, Bashir R, Britton S, Keers S, Argov Z, Mahjneh I, Fougerousse F, Beckmann JS, Bushby KM. Dysferlin is a plasma membrane protein and is expressed early in human development. Hum Mol Genet 8: 855–861, 1999. - PubMed
- Babiychuk EB, Monastyrskaya K, Potez S, Draeger A. Intracellular Ca2+ operates a switch between repair and lysis of streptolysin O-perforated cells. Cell Death Differ 16: 1126–1134, 2009. - PubMed
- Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature 423: 168–172, 2003. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources