Synergistic anti-Campylobacter jejuni activity of fluoroquinolone and macrolide antibiotics with phenolic compounds - PubMed (original) (raw)
Synergistic anti-Campylobacter jejuni activity of fluoroquinolone and macrolide antibiotics with phenolic compounds
Euna Oh et al. Front Microbiol. 2015.
Abstract
The increasing resistance of Campylobacter to clinically important antibiotics, such as fluoroquinolones and macrolides, is a serious public health problem. The objective of this study is to investigate synergistic anti-Campylobacter jejuni activity of fluoroquinolones and macrolides in combination with phenolic compounds. Synergistic antimicrobial activity was measured by performing a checkerboard assay with ciprofloxacin and erythromycin in the presence of 21 phenolic compounds. Membrane permeability changes in C. jejuni by phenolic compounds were determined by measuring the level of intracellular uptake of 1-N-phenylnaphthylamine (NPN). Antibiotic accumulation assays were performed to evaluate the level of ciprofloxacin accumulation in C. jejuni. Six phenolic compounds, including p-coumaric acid, sinapic acid, caffeic acid, vanillic acid, gallic acid, and taxifolin, significantly increased the susceptibility to ciprofloxacin and erythromycin in several human and poultry isolates. The synergistic antimicrobial effect was also observed in ciprofloxacin- and erythromycin-resistant C. jejuni strains. The phenolic compounds also substantially increased membrane permeability and antibiotic accumulation in C. jejuni. Interestingly, some phenolic compounds, such as gallic acid and taxifolin, significantly reduced the expression of the CmeABC multidrug efflux pump. Phenolic compounds increased the NPN accumulation in the cmeB mutant, indicating phenolic compounds may affect the membrane permeability. In this study, we successfully demonstrated that combinational treatment of C. jejuni with antibiotics and phenolic compounds synergistically inhibits C. jejuni by impacting both antimicrobial influx and efflux.
Keywords: Campylobacter jejuni; fluoroquinolones; macrolides; phenolic compound; synergism.
Figures
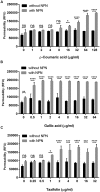
FIGURE 1
Changes in the membrane permeability of Campylobacter jejuni by _p_-coumaric acid (A), gallic acid (B), and taxifolin (C). The assay was carried out with 10 μM 1-N_-phenylnaphthylamine (NPN). Fluorescence from samples with NPN treatment reflects permeability increase, and RFU from samples without NPN shows the background fluorescence level. The results show the means and standard deviations of triplicate samples in a single experiment. The experiment was repeated three times, and all the experiments produced similar results. Statistical significance was determined with two-way ANOVA using GraphPad Prism 6 (GraphPad Software Inc., USA). ns: P > 0.5, ∗_P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001.
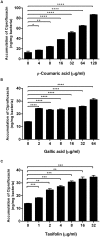
FIGURE 2
Increased accumulation of ciprofloxacin in C. jejuni by _p_-coumaric acid (A), gallic acid (B), and taxifolin (C). The results show the means and standard deviations of triplicate samples in a single experiment. Similar results were obtained in three independent experiments. Statistical analysis was carried out with the Student’s t_-test using GraphPad Prism 6 (GraphPad Software Inc.). ∗_P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001.
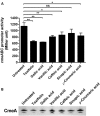
FIGURE 3
Changes in the expression levels of CmeABC by phenolic compounds. (A) Transcriptional levels of cmeABC determined by a PcmeABC::lacZ promoter fusion system with 1 μg ml-1 phenolic compounds. The results exhibit the means and standard deviations of triplicate samples in a single experiment. The experiment was repeated three times. Statistical analysis was performed with Student’s t_-test using GraphPad Prims 6 (GraphPad Software Inc.). ns: P > 0.5, ∗_P < 0.05, ∗∗P < 0.01. (B) The level of CmeA protein was demonstrated by western blotting with phenolic compounds. The CmeABC efflux pump was differentially expressed by phenolic compounds. Phenolic compounds (1 μg ml-1) were treated on each sample.
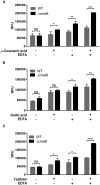
FIGURE 4
Membrane permeability changes in C. jejuni NCTC 11168 (WT) and its isogenic cmeB mutant by _p_-coumaric acid (A), gallic acid (B), and taxifolin (C). The NPN permeabilization assay was performed with 10 μM NPN. In the assay, 1 μg ml-1 phenolic compounds and 0.05 mM EDTA were used. Similar results were produced in three independent experiments. Statistical analysis was carried out with Student’s t_-test using GraphPad Prism 6 (GraphPad Software INC.). ns: P > 0.5, ∗_P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001.
Similar articles
- Fluoroquinolone and macrolide resistance in Campylobacter jejuni isolated from broiler slaughterhouses in southern Brazil.
Sierra-Arguello YM, Perdoncini G, Morgan RB, Salle CT, Moraes HL, Gomes MJ, do Nascimento VP. Sierra-Arguello YM, et al. Avian Pathol. 2016;45(1):66-72. doi: 10.1080/03079457.2015.1120272. Avian Pathol. 2016. PMID: 26925976 - [Molecular analysis of fluoroquinolones and macrolides resistance in Campylobacter jejuni isolates from humans, bovine and chicken meat].
González-Hein G, Cordero N, García P, Figueroa G. González-Hein G, et al. Rev Chilena Infectol. 2013 Apr;30(2):135-9. doi: 10.4067/S0716-10182013000200003. Rev Chilena Infectol. 2013. PMID: 23677151 Spanish. - Sensitization of Campylobacter jejuni to fluoroquinolone and macrolide antibiotics by antisense inhibition of the CmeABC multidrug efflux transporter.
Jeon B, Zhang Q. Jeon B, et al. J Antimicrob Chemother. 2009 May;63(5):946-8. doi: 10.1093/jac/dkp067. Epub 2009 Mar 11. J Antimicrob Chemother. 2009. PMID: 19279049 Free PMC article. - The Current State of Macrolide Resistance in Campylobacter spp.: Trends and Impacts of Resistance Mechanisms.
Bolinger H, Kathariou S. Bolinger H, et al. Appl Environ Microbiol. 2017 May 31;83(12):e00416-17. doi: 10.1128/AEM.00416-17. Print 2017 Jun 15. Appl Environ Microbiol. 2017. PMID: 28411226 Free PMC article. Review. - Macrolide resistance in Campylobacter jejuni and Campylobacter coli.
Gibreel A, Taylor DE. Gibreel A, et al. J Antimicrob Chemother. 2006 Aug;58(2):243-55. doi: 10.1093/jac/dkl210. Epub 2006 May 30. J Antimicrob Chemother. 2006. PMID: 16735431 Review.
Cited by
- Synergistic effect of phenylpropanoids and flavonoids with antibiotics against Gram-positive and Gram-negative bacterial strains.
Kincses A, Ghazal TSA, Hohmann J. Kincses A, et al. Pharm Biol. 2024 Dec;62(1):659-665. doi: 10.1080/13880209.2024.2389105. Epub 2024 Aug 9. Pharm Biol. 2024. PMID: 39126171 Free PMC article. - Bacterial Efflux Pump Inhibitors Reduce Antibiotic Resistance.
Zhang L, Tian X, Sun L, Mi K, Wang R, Gong F, Huang L. Zhang L, et al. Pharmaceutics. 2024 Jan 25;16(2):170. doi: 10.3390/pharmaceutics16020170. Pharmaceutics. 2024. PMID: 38399231 Free PMC article. Review. - Phenylpropanoid Derivatives and Their Role in Plants' Health and as antimicrobials.
Ortiz A, Sansinenea E. Ortiz A, et al. Curr Microbiol. 2023 Oct 20;80(12):380. doi: 10.1007/s00284-023-03502-x. Curr Microbiol. 2023. PMID: 37864088 Review. - Niosomes for Topical Application of Antioxidant Molecules: Design and In Vitro Behavior.
Sguizzato M, Pepe A, Baldisserotto A, Barbari R, Montesi L, Drechsler M, Mariani P, Cortesi R. Sguizzato M, et al. Gels. 2023 Jan 26;9(2):107. doi: 10.3390/gels9020107. Gels. 2023. PMID: 36826277 Free PMC article. - Antimicrobial Properties of Essential Oils Obtained from Autochthonous Aromatic Plants.
Boy FR, Benito MJ, Córdoba MG, Rodríguez A, Casquete R. Boy FR, et al. Int J Environ Res Public Health. 2023 Jan 17;20(3):1657. doi: 10.3390/ijerph20031657. Int J Environ Res Public Health. 2023. PMID: 36767025 Free PMC article.
References
- Bennett R. N., Rosa E. A., Ferreira-Cardoso J. V. (2009). Industrial processing effects on chestnut fruits (Castanea sativa Mill.). 2. Crude protein, free amino acids and phenolic phytochemicals. Int. J. Food Sci. Technol. 44 2613–2619. 10.1111/j.1365-2621.2009.02092.x - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources