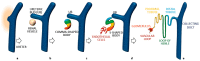Role of the Polycystins in Cell Migration, Polarity, and Tissue Morphogenesis - PubMed (original) (raw)
Review
Role of the Polycystins in Cell Migration, Polarity, and Tissue Morphogenesis
Elisa Agnese Nigro et al. Cells. 2015.
Abstract
Cystic kidney diseases (CKD) is a class of disorders characterized by ciliary dysfunction and, therefore, belonging to the ciliopathies. The prototype CKD is autosomal dominant polycystic kidney disease (ADPKD), whose mutated genes encode for two membrane-bound proteins, polycystin-1 (PC-1) and polycystin-2 (PC-2), of unknown function. Recent studies on CKD-associated genes identified new mechanisms of morphogenesis that are central for establishment and maintenance of proper renal tubular diameter. During embryonic development in the mouse and lower vertebrates a convergent-extension (CE)-like mechanism based on planar cell polarity (PCP) and cellular intercalation is involved in "sculpting" the tubules into a narrow and elongated shape. Once the appropriate diameter is established, further elongation occurs through oriented cell division (OCD). The polycystins (PCs) regulate some of these essential processes. In this review we summarize recent work on the role of PCs in regulating cell migration, the cytoskeleton, and front-rear polarity. These important properties are essential for proper morphogenesis of the renal tubules and the lymphatic vessels. We highlight here several open questions and controversies. Finally, we try to outline some of the next steps required to study these processes and their relevance in physiological and pathological conditions.
Keywords: cell migration; cell polarity; cilia; epithelial morphogenesis; planar cell polarity; polycystic kidney disease; polycystin; renal cyst.
Figures
Figure 1
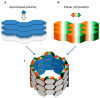
Apical-basal and Planar Polarity in Epithelia. Schematic representation of an epithelium, showing an apical-basal polarity along the vertical axis (a) and a planar cell polarity along the orthogonal axis (b); their appearance in association is shown in a tubular structure (c).
Figure 2
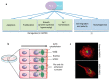
Main developmental steps of the renal nephron. Branching of the ureter generates ureteric buds (UB), surrounded by mesenchymal tissue (a); their interaction induces a mesenchymal-to-epithelial transition and generates the renal vescicle (b); that develops in a patterned comma-shaped body (c); subsequently extending in a tubular S-shaped body (d). On one side the S-shaped body makes contact with the ureter, on the other side it connects with migrating endothelial cells that will form the vascular loop within the glomerulus. The patterned expression of specific transcription factors in each tract of the S-shaped body generates the different structures of the mature nephron (see color code (e)).
Figure 3
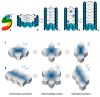
Narrowing and elongation of developing renal tubules and collecting ducts. During embryonic development, the S-shaped body tubular structure (a) elongates and establish its diameter in a proliferation-independent manner: because cells intercalate perpendicularly to the longitudinal axis, the tubule becomes narrower and longer without changing cell number (b,c); in post-natal phases, the longitudinal orientation of cell division allows to maintain the diameter and to increase length of the tubule (d,e). During cell intercalation, cells resolve from a horizontal status to a vertical one passing through an intermediate cross-shaped state (f) When re-shaping involves a larger number of cells, the intermediate status displays a rosette profile (g).
Figure 4
The Polycystins in Cell Migration. PC-1 and PC-2 are involved in different pathways and functional roles, among these they were reported to inhibit apoptosis, proliferation, and growth. Furthermore, they are known to regulate calcium homeostasis. All these processes were shown to be deregulated in ADPKD. Additional functions of the PCs more recently reported involve regulation of cell migration and tissue morphogenesis. The involvement of these last two functions in the disease remains unclear (see text) (a); schematic summary showing the cellular effects of the PCs in cell migration and front-rear polarity in wound-healing assays [5,6,8,74]. PC-1 regulates the actin and microtubular cystoskeleton and the turnover rates of FAs and AJs and affecting both the rates of cell migration and front-rear polarity (b); at a single cell level MDCKII cells appear asymmetrical, whereas overexpression of PC-1 induces elongation and cellular asymmetry with a polarized actin cytoskeleton (red) and the Golgi (green) re-positioned in front of the nucleus (blue) (c).
Similar articles
- Disruption of Core Planar Cell Polarity Signaling Regulates Renal Tubule Morphogenesis but Is Not Cystogenic.
Kunimoto K, Bayly RD, Vladar EK, Vonderfecht T, Gallagher AR, Axelrod JD. Kunimoto K, et al. Curr Biol. 2017 Oct 23;27(20):3120-3131.e4. doi: 10.1016/j.cub.2017.09.011. Epub 2017 Oct 12. Curr Biol. 2017. PMID: 29033332 Free PMC article. - Polycystin-1 binds Par3/aPKC and controls convergent extension during renal tubular morphogenesis.
Castelli M, Boca M, Chiaravalli M, Ramalingam H, Rowe I, Distefano G, Carroll T, Boletta A. Castelli M, et al. Nat Commun. 2013;4:2658. doi: 10.1038/ncomms3658. Nat Commun. 2013. PMID: 24153433 Free PMC article. - Polycystic kidney disease--the ciliary connection.
Ong AC, Wheatley DN. Ong AC, et al. Lancet. 2003 Mar 1;361(9359):774-6. doi: 10.1016/S0140-6736(03)12662-1. Lancet. 2003. PMID: 12620752 - The kidney and planar cell polarity.
Carroll TJ, Yu J. Carroll TJ, et al. Curr Top Dev Biol. 2012;101:185-212. doi: 10.1016/B978-0-12-394592-1.00011-9. Curr Top Dev Biol. 2012. PMID: 23140630 Free PMC article. Review. - Polycystins: what polycystic kidney disease tells us about sperm.
Kierszenbaum AL. Kierszenbaum AL. Mol Reprod Dev. 2004 Apr;67(4):385-8. doi: 10.1002/mrd.20042. Mol Reprod Dev. 2004. PMID: 14991728 Review.
Cited by
- Adenylyl cyclase 5 links changes in calcium homeostasis to cAMP-dependent cyst growth in polycystic liver disease.
Spirli C, Mariotti V, Villani A, Fabris L, Fiorotto R, Strazzabosco M. Spirli C, et al. J Hepatol. 2017 Mar;66(3):571-580. doi: 10.1016/j.jhep.2016.10.032. Epub 2016 Nov 5. J Hepatol. 2017. PMID: 27826057 Free PMC article. - Polycystin-1 Regulates Actomyosin Contraction and the Cellular Response to Extracellular Stiffness.
Nigro EA, Distefano G, Chiaravalli M, Matafora V, Castelli M, Pesenti Gritti A, Bachi A, Boletta A. Nigro EA, et al. Sci Rep. 2019 Nov 12;9(1):16640. doi: 10.1038/s41598-019-53061-0. Sci Rep. 2019. PMID: 31719603 Free PMC article. - Interleukin-1 receptor activation aggravates autosomal dominant polycystic kidney disease by modulating regulated necrosis.
Yang B, Fu L, Privratsky JR, Lu X, Ren J, Mei C, Crowley SD. Yang B, et al. Am J Physiol Renal Physiol. 2019 Aug 1;317(2):F221-F228. doi: 10.1152/ajprenal.00104.2019. Epub 2019 May 29. Am J Physiol Renal Physiol. 2019. PMID: 31141402 Free PMC article. - Disruption of Core Planar Cell Polarity Signaling Regulates Renal Tubule Morphogenesis but Is Not Cystogenic.
Kunimoto K, Bayly RD, Vladar EK, Vonderfecht T, Gallagher AR, Axelrod JD. Kunimoto K, et al. Curr Biol. 2017 Oct 23;27(20):3120-3131.e4. doi: 10.1016/j.cub.2017.09.011. Epub 2017 Oct 12. Curr Biol. 2017. PMID: 29033332 Free PMC article. - The Sorting Nexin 3 Retromer Pathway Regulates the Cell Surface Localization and Activity of a Wnt-Activated Polycystin Channel Complex.
Feng S, Streets AJ, Nesin V, Tran U, Nie H, Onopiuk M, Wessely O, Tsiokas L, Ong ACM. Feng S, et al. J Am Soc Nephrol. 2017 Oct;28(10):2973-2984. doi: 10.1681/ASN.2016121349. Epub 2017 Jun 15. J Am Soc Nephrol. 2017. PMID: 28620080 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous