Omega-3 fatty acids for depression in adults - PubMed (original) (raw)
Review
Omega-3 fatty acids for depression in adults
Katherine M Appleton et al. Cochrane Database Syst Rev. 2015.
Update in
- Omega-3 fatty acids for depression in adults.
Appleton KM, Voyias PD, Sallis HM, Dawson S, Ness AR, Churchill R, Perry R. Appleton KM, et al. Cochrane Database Syst Rev. 2021 Nov 24;11(11):CD004692. doi: 10.1002/14651858.CD004692.pub5. Cochrane Database Syst Rev. 2021. PMID: 34817851 Free PMC article. Review.
Abstract
Background: Major depressive disorder (MDD) is highly debilitating, difficult to treat, has a high rate of recurrence, and negatively impacts the individual and society as a whole. One emerging potential treatment for MDD is n-3 polyunsaturated fatty acids (n-3PUFAs), also known as omega-3 oils, naturally found in fatty fish, some other seafood, and some nuts and seeds. Various lines of evidence suggest a role for n-3PUFAs in MDD, but the evidence is far from conclusive. Reviews and meta-analyses clearly demonstrate heterogeneity between studies. Investigations of heterogeneity suggest differential effects of n-3PUFAs, depending on severity of depressive symptoms, where no effects of n-3PUFAs are found in studies of individuals with mild depressive symptomology, but possible benefit may be suggested in studies of individuals with more severe depressive symptomology.
Objectives: To assess the effects of n-3 polyunsaturated fatty acids (also known as omega-3 fatty acids) versus a comparator (e.g. placebo, anti-depressant treatment, standard care, no treatment, wait-list control) for major depressive disorder (MDD) in adults.
Search methods: We searched the Cochrane Depression, Anxiety and Neurosis Review Group's Specialised Registers (CCDANCTR) and International Trial Registries over all years to May 2015. We searched the database CINAHL over all years of records to September 2013.
Selection criteria: We included studies in the review if they: were a randomised controlled trial; provided n-3PUFAs as an intervention; used a comparator; measured depressive symptomology as an outcome; and were conducted in adults with MDD. Primary outcomes were depressive symptomology (continuous data collected using a validated rating scale) and adverse events. Secondary outcomes were depressive symptomology (dichotomous data on remission and response), quality of life, and failure to complete studies.
Data collection and analysis: We used standard methodological procedures as expected by Cochrane.
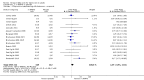
Main results: We found 26 relevant studies: 25 studies involving a total of 1438 participants investigated the impact of n-3PUFA supplementation compared to placebo, and one study involving 40 participants investigated the impact of n-3PUFA supplementation compared to antidepressant treatment.For the placebo comparison, n-3PUFA supplementation results in a small to modest benefit for depressive symptomology, compared to placebo: standardised mean difference (SMD) -0.32 (95% confidence interval (CI) -0.12 to -0.52; 25 studies, 1373 participants, very low quality evidence), but this effect is unlikely to be clinically meaningful (an SMD of 0.32 represents a difference between groups in scores on the HDRS (17-item) of approximately 2.2 points (95% CI 0.8 to 3.6)). The confidence intervals include both a possible clinically important effect and a possible negligible effect, and there is considerable heterogeneity between the studies. Although the numbers of individuals experiencing adverse events were similar in intervention and placebo groups (odds ratio (OR) 1.24, 95% CI 0.95 to 1.62; 19 studies, 1207 participants; very low-quality evidence), the confidence intervals include a significant increase in adverse events with n-3PUFAs as well as a small possible decrease. Rates of remission and response, quality of life, and rates of failure to complete studies were also similar between groups, but confidence intervals are again wide.The evidence on which these results are based is very limited. All studies contributing to our analyses were of direct relevance to our research question, but we rated the quality of the evidence for all outcomes as low to very low. The number of studies and number of participants contributing to all analyses were low, and the majority of studies were small and judged to be at high risk of bias on several measures. Our analyses were also likely to be highly influenced by three large trials. Although we judge these trials to be at low risk of bias, they contribute 26.9% to 82% of data. Our effect size estimates are also imprecise. Funnel plot asymmetry and sensitivity analyses (using fixed-effect models, and only studies judged to be at low risk of selection bias, performance bias or attrition bias) also suggest a likely bias towards a positive finding for n-3PUFAs. There was substantial heterogeneity in analyses of our primary outcome of depressive symptomology. This heterogeneity was not explained by the presence or absence of comorbidities or by the presence or absence of adjunctive therapy.Only one study was available for the antidepressant comparison, involving 40 participants. This study found no differences between treatment with n-3PUFAs and treatment with antidepressants in depressive symptomology (mean difference (MD) -0.70 (95% CI -5.88 to 4.48)), rates of response to treatment or failure to complete. Adverse events were not reported in a manner suitable for analysis, and rates of depression remission and quality of life were not reported.
Authors' conclusions: At present, we do not have sufficient high quality evidence to determine the effects of n-3PUFAs as a treatment for MDD. Our primary analyses suggest a small-to-modest, non-clinically beneficial effect of n-3PUFAs on depressive symptomology compared to placebo; however the estimate is imprecise, and we judged the quality of the evidence on which this result is based to be low/very low. Sensitivity analyses, funnel plot inspection and comparison of our results with those of large well-conducted trials also suggest that this effect estimate is likely to be biased towards a positive finding for n-3PUFAs, and that the true effect is likely to be smaller. Our data, however, also suggest similar rates of adverse events and numbers failing to complete trials in n-3PUFA and placebo groups, but again our estimates are very imprecise. The one study that directly compares n-3PUFAs and antidepressants in our review finds comparable benefit. More evidence, and more complete evidence, are required, particularly regarding both the potential positive and negative effects of n-3PUFAs for MDD.
Conflict of interest statement
KA: None known HS: None known RP: None known AN: None known RC: None known
Figures
Figure 1
PRISMA Diagram
Figure 2
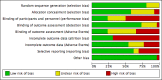
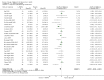
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3
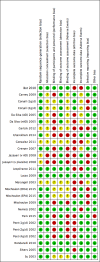
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Figure 4
Forest plot of comparison: 1 n‐3PUFAs vs placebo, outcome: 1.1 Depressive symptomology (continuous).
Figure 5
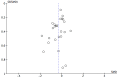
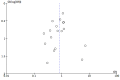
Funnel plot of comparison: 1 n‐3PUFAs vs placebo, outcome: 1.1 Depressive symptomology (continuous).
Figure 6
Forest plot of comparison: 1 n‐3PUFAs vs placebo, outcome: 1.2 Adverse events.
Figure 7
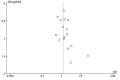
Funnel plot of comparison: 1 n‐3PUFAs vs Placebo, outcome: 1.2 Adverse events.
Figure 8
Forest plot of comparison: 1 n‐3PUFAs vs placebo, outcome: 1.3 Depressive symptomology (dichotomous data): remission.
Figure 9
Forest plot of comparison: 1 n‐3PUFAs vs placebo, outcome: 1.4 Depressive symptomology (dichotomous data): response.
Figure 10
Forest plot of comparison: 1 n‐3PUFAs vs placebo, outcome: 1.5 Quality of life.
Figure 11
Forest plot of comparison: 1 n‐3PUFAs vs placebo, outcome: 1.6 Failure to complete.
Figure 12
Funnel plot of comparison: 1 n‐3PUFAs vs Placebo, outcome: 1.6 Failure to complete.
Figure 13
Forest plot of comparison: 1 n‐3PUFAs vs placebo, outcome: 3.1 Depressive symptomology (continuous): Sub‐groups based on presence / absence of comorbidities.
Figure 14
Forest plot of comparison: 1 n‐3PUFAs vs placebo, outcome: 4.1 Depressive symptomology (continuous): Sub‐groups based on use / non‐use of adjunctive therapies.
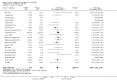
Analysis 1.1
Comparison 1 n‐3PUFAs vs placebo, Outcome 1 Depressive symptomology (continuous).
Analysis 1.2
Comparison 1 n‐3PUFAs vs placebo, Outcome 2 Adverse events.
Analysis 1.3
Comparison 1 n‐3PUFAs vs placebo, Outcome 3 Depressive symptomology (dichotomous ‐ remission).
Analysis 1.4
Comparison 1 n‐3PUFAs vs placebo, Outcome 4 Depressive symptomology (dichotomous ‐ response).
Analysis 1.5
Comparison 1 n‐3PUFAs vs placebo, Outcome 5 Quality of life.
Analysis 1.6
Comparison 1 n‐3PUFAs vs placebo, Outcome 6 Failure to complete.
Analysis 2.1
Comparison 2 n‐3PUFAs vs antidepressant, Outcome 1 Depressive symptomology (continuous).
Analysis 2.2
Comparison 2 n‐3PUFAs vs antidepressant, Outcome 2 Depressive symptomology (dichotomous ‐ response).
Analysis 2.3
Comparison 2 n‐3PUFAs vs antidepressant, Outcome 3 Failure to complete.
Analysis 3.1
Comparison 3 Subgroup analyses ‐ n‐3PUFAs vs placebo ‐ analyses based on comorbidities, Outcome 1 Depressive symptomology (continuous).
Analysis 3.2
Comparison 3 Subgroup analyses ‐ n‐3PUFAs vs placebo ‐ analyses based on comorbidities, Outcome 2 Adverse events.
Analysis 4.1
Comparison 4 Subgroup analyses: n‐3PUFAs vs placebo ‐ analyses based on adjunctive therapy, Outcome 1 Depressive symptomology (continuous).
Analysis 4.2
Comparison 4 Subgroup analyses: n‐3PUFAs vs placebo ‐ analyses based on adjunctive therapy, Outcome 2 Adverse events.
Similar articles
- Omega-3 fatty acids for depression in adults.
Appleton KM, Voyias PD, Sallis HM, Dawson S, Ness AR, Churchill R, Perry R. Appleton KM, et al. Cochrane Database Syst Rev. 2021 Nov 24;11(11):CD004692. doi: 10.1002/14651858.CD004692.pub5. Cochrane Database Syst Rev. 2021. PMID: 34817851 Free PMC article. Review. - ω-3 Fatty acids for major depressive disorder in adults: an abridged Cochrane review.
Appleton KM, Sallis HM, Perry R, Ness AR, Churchill R. Appleton KM, et al. BMJ Open. 2016 Mar 2;6(3):e010172. doi: 10.1136/bmjopen-2015-010172. BMJ Open. 2016. PMID: 26936905 Free PMC article. Review. - Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article. - Vortioxetine for depression in adults.
Koesters M, Ostuzzi G, Guaiana G, Breilmann J, Barbui C. Koesters M, et al. Cochrane Database Syst Rev. 2017 Jul 5;7(7):CD011520. doi: 10.1002/14651858.CD011520.pub2. Cochrane Database Syst Rev. 2017. PMID: 28677828 Free PMC article. Review. - Antidepressants for the treatment of depression in people with cancer.
Ostuzzi G, Matcham F, Dauchy S, Barbui C, Hotopf M. Ostuzzi G, et al. Cochrane Database Syst Rev. 2015 Jun 1;2015(6):CD011006. doi: 10.1002/14651858.CD011006.pub2. Cochrane Database Syst Rev. 2015. PMID: 26029972 Free PMC article. Updated. Review.
Cited by
- Self-care for anxiety and depression: a comparison of evidence from Cochrane reviews and practice to inform decision-making and priority-setting.
Pilkington K, Wieland LS. Pilkington K, et al. BMC Complement Med Ther. 2020 Aug 10;20(1):247. doi: 10.1186/s12906-020-03038-8. BMC Complement Med Ther. 2020. PMID: 32778171 Free PMC article. - Biotechnological production of omega-3 fatty acids: current status and future perspectives.
Qin J, Kurt E, LBassi T, Sa L, Xie D. Qin J, et al. Front Microbiol. 2023 Nov 7;14:1280296. doi: 10.3389/fmicb.2023.1280296. eCollection 2023. Front Microbiol. 2023. PMID: 38029217 Free PMC article. Review. - Anti-Inflammatory Treatment Efficacy in Major Depressive Disorder: A Systematic Review of Meta-Analyses.
Simon MS, Arteaga-Henríquez G, Fouad Algendy A, Siepmann T, Illigens BMW. Simon MS, et al. Neuropsychiatr Dis Treat. 2023 Jan 5;19:1-25. doi: 10.2147/NDT.S385117. eCollection 2023. Neuropsychiatr Dis Treat. 2023. PMID: 36636142 Free PMC article. Review. - Association of Use of Omega-3 Polyunsaturated Fatty Acids With Changes in Severity of Anxiety Symptoms: A Systematic Review and Meta-analysis.
Su KP, Tseng PT, Lin PY, Okubo R, Chen TY, Chen YW, Matsuoka YJ. Su KP, et al. JAMA Netw Open. 2018 Sep 7;1(5):e182327. doi: 10.1001/jamanetworkopen.2018.2327. JAMA Netw Open. 2018. PMID: 30646157 Free PMC article. - The efficacy of fish oil supplements in the treatment of depression: food for thought.
Bastiaansen JA, Munafò MR, Appleton KM, Oldehinkel AJ. Bastiaansen JA, et al. Transl Psychiatry. 2016 Dec 6;6(12):e975. doi: 10.1038/tp.2016.243. Transl Psychiatry. 2016. PMID: 27922634 Free PMC article. No abstract available.
References
References to studies included in this review
- Bot M, Pouwer F, Assies J, Jansen E, Beekman ATF, Jonge P. Supplementation with eicosapentaenoic omega‐3 fatty acid does not influence serum brain‐derived neurotrophic factor in diabetes mellitus patients with major depression: A randomized controlled pilot study. Neuropsychobiology 2011;63(4):219‐23. [] - PubMed
- Bot M, Pouwer F, Assies J, Jansen E, Diamant M, Snoek FJ, et al. Eicosapentaenoic acid as an add‐on to antidepressant medication for co‐morbid major depression in patients with diabetes mellitus: A randomized, double‐blind placebo‐controlled study [ISRCTN30877831; NTR624]. Journal of Affective Disorders 2010;126(1‐2):282‐6. [] - PubMed
- Mocking RJ, Assies J, Bot M, Jansen E, Schene AH, Pouwer F. Biological effects of add‐on eicosapentaenoic acid supplementation in diabetes mellitus and co‐morbid depression: a randomized controlled trial. PloS One 2012;7(11):e49431. [] - PMC - PubMed
- Pouwer F. Addition of eicosapentaenoic acid to maintenance anti‐depressant therapy in diabetes patients with major depressive disorder: a double‐blind, placebo‐controlled pilot study. www.controlled‐trials.com/ISRCTN30877831 (Accessed 21 August 2014). []
- 3000967
- Anonymous. News breaks. Nutrition Today 2009;44(6):234‐5. []
- Anonymous. Use of omega‐3 with treatment for depression in heart disease patients may not provide benefit. Nurse Prescribing 2009;7(11):523‐4. []
- Bot M, Carney RM, Freedland KE, Rubin EH, Rich MW, Steinmeyer BC, et al. Inflammation and treatment response to sertraline in patients with coronary heart disease and comorbid major depression. Journal of Psychosomatic Research 2011;71(1):13‐7. [] - PMC - PubMed
- Carney RM. Omega‐3 fatty acids to improve depression and reduce cardiovascular risk factors. <ClinicalTrials.gov/show/NCT00116857> (Accessed 21 August 2014). []
- Carney RM, Freedland KE, Rubin EH, Rich MW, Steinmeyer MS, Harris WS. Omega‐3 augmentation of sertraline in treatment of depression in patients with coronary heart disease: A randomized controlled trial [NCT00116857]. JAMA 2009;302(15):1651‐7. [] - PMC - PubMed
- Carney RM, Freedland KE, Steinmeyer BC. 'Omega‐3 fatty acids for CHD with depression': Reply to comment. JAMA 2010;303(9):836. [] - PubMed
- D'Anci KE. Nutrition updates. Nutrition Reviews 2010;68(1):71‐3. [] - PubMed
- Roest AM, Carney RM, Stein PK, Freedland KE, Meyer H, Steinmeyer BC, et al. Obstructive sleep apnea/hypopnea syndrome and poor response to sertraline in patients with coronary heart disease. Journal of Clinical Psychiatry 2012;73(1):31‐6. [] - PMC - PubMed
- 3000972
- Coryell WH. Essential fatty acids for major depression. <ClinicalTrials.gov/show/NCT00256412> (Accessed 22/07/2014). []
- Fiedorowicz JG, Hale N, Spector AA, Coryell WH. Neuroticism but not omega‐3 fatty acid levels correlate with early responsiveness to escitalopram. Annals of Clinical Psychiatry 2010;22(3):157‐63. [] - PMC - PubMed
- 3000981
- Coryell WH. Essential fatty acids for major depression. <ClinicalTrials.gov/show/NCT00256412> (Accessed 22/07/2014). []
- Fiedorowicz JG, Hale N, Spector AA, Coryell WH. Neuroticism but not omega‐3 fatty acid levels correlate with early responsiveness to escitalopram. Annals of Clinical Psychiatry 2010;22(3):157‐63. [] - PMC - PubMed
- 3000984
- Silva TM, Munhoz RP, Alvarez C, Naliwaiko K, Kiss A, Andreatini R, et al. Depression in Parkinson's disease: a double‐blind, randomized, placebo‐controlled pilot study of omega‐3 fatty‐acid supplementation. Journal of Affective Disorders 2008;111(2‐3):351‐9. [] - PubMed
- 3000987
References to studies excluded from this review
- Clayton A. Study of supplementation of antidepressants with fish oil to improve time to clinical response (SADFAT). <ClinicalTrials.gov/show/NCT00963196> (Accessed 11 September 2014). []
- 3001063
References to studies awaiting assessment
- EUCTR2006‐004949‐41‐IT. Randomized double‐blind study to evaluate the adjuvant effect of polyunsaturated fatty acids omega‐3 in therapy with SSRI paroxetine mesylate in unipolar mood depression and recurrent depression. www.ClinicalTrialsregister.eu/ctr‐search/trial/2006‐004949‐41/IT (Accessed 22nd July 2014). []
- 3001065
- Kwak KP, Lee KH. A comparative study of addition therapy of choline alfoscerate and omega 3 fatty acid in older depressed patients with or without executive dysfunction. International Psychogeriatrics 2014;25:S75‐192. []
- 3001067
- Lima L. A randomised study on the antidepressive effect of fluoxetine and folic acid, as possible augmenter, and the SYNThesis of serotonin (5‐HT) in lymphocytes prior and after treatment. <isrctn.org/ISRCTN06123818> (Accessed 26th June 2015). []
- 3001069
- Murck H. A multicentre, double‐blind, randomised, parallel group, placebo‐controlled trial of Lax‐101 (ethyl eicosapentaenoate) as adjunct therapy in patients who remain depressed following treatment with standard antidepressant therapy. 2004. []
- 3001071
- Naqvi S. Treating adolescent depression with fish oils. <ClinicalTrials.gov/show/NCT00658476> (Accessed 26th June 2015). []
- 3001073
References to ongoing studies
- Amminger GP, Rice S. Youth Depression Alleviation: a randomised controlled trial of omega‐3 fatty acids (fish oil) for major depressive disorder in young people (YoDA‐F). www.anzctr.org.au/ACTRN12613001352796.aspx (Accessed 26th June 2015). []
- Rice SM, Hickie IB, Yung AR, Mackinnon A, Berk M, Davey C, et al. Youth depression alleviation: the Fish Oil Youth Depression Study (YoDA‐F): A randomized, double‐blind, placebo‐controlled treatment trial. 13 Aug 2014 [Epub ahead of print]. [] - PubMed
- 3001084
- Carney RM. Omega‐3 for depression and other cardiac risk factors ‐ 2. <ClinicalTrials.gov/show/NCT02021669> (Accessed 26th June 2015). []
- 3001087
- Gabbay V. Omega‐3 fatty acids in adolescent depression. <ClinicalTrials.gov/show/NCT00312897> (Accessed 26th June 2015). []
- Gabbay V. The role of omega‐3 fatty acids In adolescent depression. <ClinicalTrials.gov/show/NCT00962598> (Accessed 26th June 2015). []
- 3001089
- Howe P. Fish oil treatment for depression in cardiovascular disease. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=82950 ([Accessed 26th June 2015). []
- 3001092
- Jiang W. Omega 3 for treatment of depression in patients with heart failure (OCEAN). <ClinicalTrials.gov/show/NCT02057406> (Accessed 26th June 2015). []
- 3001094
Additional references
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. Washington, DC: American Psychiatric Association, 2013.
- Appleton KM, Hayward RC, Gunnell D, Peters TJ, Rogers PJ, Kessler, D, et al. Effects of n3 long chain polyunsaturated fatty acids on depressed mood: systematic review of published trials. American Journal of Clinical Nutrition 2006;84(6):1308‐16. - PubMed
- Appleton KM, Peters TJ, Hayward RC, Heatherley SV, McNaughton SA, Rogers PJ, et al. Depressed mood and n‐3 polyunsaturated fatty acid intake from fish: non‐linear or confounded association?. Social Psychiatry and Psychiatric Epidemiology 2007;42(2):100‐4. - PubMed
- Appleton KM, Gunnell D, Peters TJ, Ness AR, Kessler D, Rogers PJ. No clear evidence of an associations between plasma concentrations of n‐3 long chain polyunsaturated fatty acids and depressed mood in a non‐clinical population. Prostaglandins, Leukotrienes and Essential Fatty Acids 2008;78:337‐42. - PubMed
- Appleton KM, Rogers PJ, Ness AR. Is there a role for n‐3 long‐chain polyunsaturated fatty acids in the regulation of mood and behaviour? A review of the evidence to date from epidemiological studies, clinical studies and intervention trials. Nutrition Research Reviews 2008;21(1):13‐41. - PubMed
References to other published versions of this review
- Silvers KM, Hackett ML, Scott KM. Omega 3 fatty acids for depression. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD004692.pub2] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical