Progesterone Enhanced Remyelination in the Mouse Corpus Callosum after Cuprizone Induced Demyelination - PubMed (original) (raw)
Progesterone Enhanced Remyelination in the Mouse Corpus Callosum after Cuprizone Induced Demyelination
Iraj Ragerdi Kashani PhD et al. Iran J Med Sci. 2015 Nov.
Abstract
Background: Progesterone as a sex steroid hormone is thought to affect and prevent demyelination, but its role in promoting myelin repair is far less investigated. In this study, remyelinating potential of progesterone in corpus callosum was evaluated on an experimental model of MS.
Methods: In this experimental study, adult male C57BL/6 mice were fed with 0.2% (w/w) cuprizone in ground breeder chow ad libitum for 6 weeks. At day zero, after cuprizone removal, mice were divided randomly into two groups: (a) placebo group, which received saline pellet implant, (b) progesterone group, which received progesterone pellet implant. Some mice of the same age were fed with their normal diet to serve as the healthy control group. Two weeks after progesterone administration, Myelin content was assessed by Luxol-fast blue staining. The myelin basic protein (MBP) and proteolipid protein (PLP) expression were assessed using Western blot analysis and the changes in the number of oligodendrocytes and oligodendroglial progenitor cells were assessed by immunohistochemistry (IHC) and flow cytometry.
Results: Luxol-fast blue staining revealed enhanced remyelination in the progesterone group when compared with the placebo group. Densitometry measurements of immunoblots demonstrated that MBP and PLP proteins contents were significantly increased in the progesterone group compared with the placebo group. Flow cytometry and IHC analysis showed increases in Olig2 and O4 cells in the progesterone group compared with the placebo group.
Conclusion: Overall, our results indicate that progesterone treatment can stimulate myelin production and that it may provide a feasible and practical way for remyelination in diseases such as multiple sclerosis.
Keywords: Multiple sclerosis; Progesterone; Remyelination.
Figures
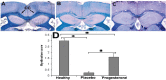
Figure 1
Remyelination in the corpus callosum two weeks after progesterone administration. Myelin content was evaluated by luxol fast blue staining. The photomicrographs were taken from sagital sections of body of corpus callosum two weeks after treatment. Corpus callosum (CC) of a healthy control group (A), placebo group (B), and progesterone implantation group (C). Quantification of myelin content in the body of corpus callosum (D) shows that in progesterone pellet implanted mice there was a further significant increase in the remyelination score compared with saline pellet implanted (placebo) mice. Data are represented as mean±SD (*P<0.05).
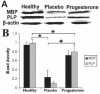
Figure 2
Protein expression of myelin basic protein (MBP) and proteolipid protein (PLP) were measured by Western blot and β actin was used as housekeeping control. A: The representative Western blot pictures of MBP and PLP protein in corpus callosum of the healthy control, placebo and progesterone administration mice. B: Bar chart showing the relative quantities of MBP and PLP measured densitometrically from the Western blots in different groups. The value represented here as mean±SEM of 3 mice in each group (*P<0.05).
Figure 3
Effects of progesterone treatment on the number of olig2 positive cells (oligodendroglial progenitor cell) in the corpus callosum of cuprizone induced demyelination mice after two weeks of injury. Light photomicrographs of immunohistochemistry with anti-olig2 antibody (arrows) in healthy control group (A), placebo group (B), and progesterone implantation group (C). The cell counting of olig2 positive cells in body of the corpus callosum (D) shows that in progesterone pellet implanted mice there was a further significant increase in the number of olig2+ cells, compared with the healthy control and saline pellet implanted (placebo) mice. The value represented here as mean±SEM of 3 mice in each group (*P<0.05). Scale bars: 100 µ.
Figure 4
Oligodendrocyte and oligodendroglial progenitor cell numbers are markedly increased in the corpus callosum of progesterone receiving mice. Mononuclear cells were isolated from corpus callosum and the frequencies of Olig2 positive cells (oligodendroglial progenitor cell) and O4 positive cells (oligodendrocyte) determined using flow cytometry two weeks after treatment. Data shown in the Figure A are representative of the three performed independent experiments. The total number of O4 positive cells, shown in B, and the total number of Olig2 positive cells, shown in C of the healthy control, placebo, and progesterone-receiving mice are shown. The data are presented as the average of the cell counts from three mice per group ±SEM in B and C (*P<0.05).
Similar articles
- Promotion of remyelination by adipose mesenchymal stem cell transplantation in a cuprizone model of multiple sclerosis.
Hedayatpour A, Ragerdi I, Pasbakhsh P, Kafami L, Atlasi N, Pirhajati Mahabadi V, Ghasemi S, Reza M. Hedayatpour A, et al. Cell J. 2013 Summer;15(2):142-51. Epub 2013 Jul 2. Cell J. 2013. PMID: 23862116 Free PMC article. - Sequential myelin protein expression during remyelination reveals fast and efficient repair after central nervous system demyelination.
Lindner M, Heine S, Haastert K, Garde N, Fokuhl J, Linsmeier F, Grothe C, Baumgärtner W, Stangel M. Lindner M, et al. Neuropathol Appl Neurobiol. 2008 Feb;34(1):105-14. doi: 10.1111/j.1365-2990.2007.00879.x. Epub 2007 Oct 24. Neuropathol Appl Neurobiol. 2008. PMID: 17961136 - 17β-Estradiol enhances the efficacy of adipose-derived mesenchymal stem cells on remyelination in mouse model of multiple sclerosis.
Ragerdi Kashani I, Hedayatpour A, Pasbakhsh P, Kafami L, Atlasi N, Pirhajati Mahabadi V, Mamoudi R, Baazm M. Ragerdi Kashani I, et al. Acta Med Iran. 2012;50(12):789-97. Acta Med Iran. 2012. PMID: 23456519 - Electroacupuncture Promotes Remyelination after Cuprizone Treatment by Enhancing Myelin Debris Clearance.
Zhu K, Sun J, Kang Z, Zou Z, Wu G, Wang J. Zhu K, et al. Front Neurosci. 2017 Jan 10;10:613. doi: 10.3389/fnins.2016.00613. eCollection 2016. Front Neurosci. 2017. PMID: 28119561 Free PMC article. - 17beta-estradiol and progesterone prevent cuprizone provoked demyelination of corpus callosum in male mice.
Acs P, Kipp M, Norkute A, Johann S, Clarner T, Braun A, Berente Z, Komoly S, Beyer C. Acs P, et al. Glia. 2009 Jun;57(8):807-14. doi: 10.1002/glia.20806. Glia. 2009. PMID: 19031445
Cited by
- SeXX Matters in Multiple Sclerosis.
Gilli F, DiSano KD, Pachner AR. Gilli F, et al. Front Neurol. 2020 Jul 3;11:616. doi: 10.3389/fneur.2020.00616. eCollection 2020. Front Neurol. 2020. PMID: 32719651 Free PMC article. Review. - Effect of Multiple Intraperitoneal Injections of Human Bone Marrow Mesenchymal Stem Cells on Cuprizone Model of Multiple Sclerosis.
Marzban M, Mousavizadeh K, Bakhshayesh M, Vousooghi N, Vakilzadeh G, Torkaman-Boutorabi A. Marzban M, et al. Iran Biomed J. 2018 Sep;22(5):312-21. doi: 10.29252/ibj.22.5.312. Epub 2018 Feb 7. Iran Biomed J. 2018. PMID: 29409311 Free PMC article. - Sex Steroids, Adult Neurogenesis, and Inflammation in CNS Homeostasis, Degeneration, and Repair.
Larson TA. Larson TA. Front Endocrinol (Lausanne). 2018 Apr 30;9:205. doi: 10.3389/fendo.2018.00205. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29760681 Free PMC article. Review. - Olig2+ single-colony-derived cranial bone-marrow mesenchymal stem cells achieve improved regeneration in a cuprizone-induced demyelination mouse model.
Peng D, Lu R, Lü L, Yao Q, Yang K, Xu Y, Feng X, Pan R, Ma Y. Peng D, et al. J Zhejiang Univ Sci B. 2024 Sep 25;25(12):1108-1114. doi: 10.1631/jzus.B2300790. J Zhejiang Univ Sci B. 2024. PMID: 39743297 Free PMC article. - The Effects of Neuroactive Steroids on Myelin in Health and Disease.
Kalakh S, Mouihate A. Kalakh S, et al. Med Princ Pract. 2024;33(3):198-214. doi: 10.1159/000537794. Epub 2024 Feb 13. Med Princ Pract. 2024. PMID: 38350432 Free PMC article. Review.
References
- Crawford DK, Mangiardi M, Song B, Patel R, Du S, Sofroniew MV, et al. Oestrogen receptor beta ligand: a novel treatment to enhance endogenous functional remyelination. Brain. 2010;133:2999–3016. doi: 10.1093/brain/awq237. [ PMC Free Article] - DOI - PMC - PubMed
- Ibanez C, Shields SA, El-Etr M, Baulieu EE, Schumacher M, Franklin RJ. Systemic progesterone administration results in a partial reversal of the age-associated decline in CNS remyelination following toxin-induced demyelination in male rats. Neuropathol Appl Neurobiol. 2004;30:80–9. doi: 10.1046/j.0305-1846.2003.00515.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous