Dental Pulp Defence and Repair Mechanisms in Dental Caries - PubMed (original) (raw)
Review
Dental Pulp Defence and Repair Mechanisms in Dental Caries
Jean-Christophe Farges et al. Mediators Inflamm. 2015.
Abstract
Dental caries is a chronic infectious disease resulting from the penetration of oral bacteria into the enamel and dentin. Microorganisms subsequently trigger inflammatory responses in the dental pulp. These events can lead to pulp healing if the infection is not too severe following the removal of diseased enamel and dentin tissues and clinical restoration of the tooth. However, chronic inflammation often persists in the pulp despite treatment, inducing permanent loss of normal tissue and reducing innate repair capacities. For complete tooth healing the formation of a reactionary/reparative dentin barrier to distance and protect the pulp from infectious agents and restorative materials is required. Clinical and in vitro experimental data clearly indicate that dentin barrier formation only occurs when pulp inflammation and infection are minimised, thus enabling reestablishment of tissue homeostasis and health. Therefore, promoting the resolution of pulp inflammation may provide a valuable therapeutic opportunity to ensure the sustainability of dental treatments. This paper focusses on key cellular and molecular mechanisms involved in pulp responses to bacteria and in the pulpal transition between caries-induced inflammation and dentinogenic-based repair. We report, using selected examples, different strategies potentially used by odontoblasts and specialized immune cells to combat dentin-invading bacteria in vivo.
Figures
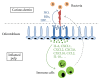
Figure 1
Two key aspects of the odontoblast defence against dentin-invading bacteria. Bacteria (B) present in the carious dentinal lesion release pathogenic components that activate (blue arrow) odontoblasts (dark blue) adjacent to the lesion, triggering the production of antibacterial molecules (blue dots). These molecules diffuse through dentin tubules in an attempt to destroy the invading microorganisms (NO, BDs) or considerably decrease their pathogenicity (LBP). In parallel, proinflammatory and immunomodulatory mediators (green dots), including IL-6, IL-10, CXCL1, CXCL2, CXCL8 (IL-8), CXCL10, and CCL2, are secreted by odontoblasts at the opposite cell pole and diffuse into the subodontoblast pulp area (green arrow) where they activate and mobilize various populations of immune cells (as described in the main text body) enabling the immunosurveillance of the tissue. Immune cells then migrate (dotted grey arrow) towards the pulp-dentin interface beneath the lesion to combat the bacteria and coordinate the immune defense response.
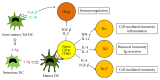
Figure 2
The putative role of dendritic cells (DCs) in the regulation of T helper (Th) and induced regulatory T (iTreg) cell differentiation. Upon encountering antigens (Ag), immature DCs usually become mature DCs which present antigens to naive CD4+ (Th0) cells. Upon antigen recognition, Th0 cells clonally expand and can differentiate into various subsets of effector cells (Th1, Th2, or Th17) or into iTreg cells depending on the cytokines present in their environment. Alternatively, immature DCs can mature only partially to become Tolerogenic-DCs (Tol-DCs) which can directly induce iTreg cell differentiation through TGF-β and IL-10 secretion. IL, interleukin; IFN, interferon; TGF, transforming growth factor; Ig, immunoglobulin.
Figure 3
Tables ((a) and (b)) showing the key functions associated with the 16 and 3 molecular networks identified as being significantly activated (≥ 6 focus genes) in carious and healthy pulpal tissue, respectively. Shading of boxes indicates the networks which associated with the function and hence supported its inclusion as being active. Analysis was performed using the Ingenuity Pathways Analysis (IPA) software (
http://www.ingenuity.com/products/ipa
) on the high-throughput datasets reported in McLachlan et al. [11]. Sixteen and three functional categories were identified as being activated in carious diseased and healthy pulpal tissues, respectively. Carious diseased pulp tissue clearly demonstrated increased molecular network and functional activity compared with healthy pulpal tissue. Asterisks (∗) in (a) indicate functions which are associated with immune system cells (as identified by IPA); notably some evidence of hard tissue repair function was also evident (#). Ontological functions identified in (b) likely associate with pulp tissue homeostatic processes. Image (c) shows an example network (network 1 from the carious pulp tissue dataset) which also shows the subcellular localisation of the molecules that were identified as differentially expressed. The activation of this network via intracellular signalling cascades results in the elaboration of key inflammatory-associated chemokines, such as CXCL8 (IL-8) and CCL2, and the matrix metalloproteinases (MMPs) 1 and 9.
Similar articles
- Is hard tissue formation in the dental pulp after the death of the primary odontoblasts a regenerative or a reparative process?
Ricucci D, Loghin S, Lin LM, Spångberg LS, Tay FR. Ricucci D, et al. J Dent. 2014 Sep;42(9):1156-70. doi: 10.1016/j.jdent.2014.06.012. Epub 2014 Jul 5. J Dent. 2014. PMID: 25008021 - Pulp and apical tissue response to deep caries in immature teeth: A histologic and histobacteriologic study.
Ricucci D, Siqueira JF Jr, Loghin S, Lin LM. Ricucci D, et al. J Dent. 2017 Jan;56:19-32. doi: 10.1016/j.jdent.2016.10.005. Epub 2016 Oct 12. J Dent. 2017. PMID: 27744048 - On the repair of the dentine barrier.
Fransson H. Fransson H. Swed Dent J Suppl. 2012;(226):9-84. Swed Dent J Suppl. 2012. PMID: 22834214 - Pulp-dentin biology in restorative dentistry. Part 4: Dental caries--characteristics of lesions and pulpal reactions.
Bjørndal L, Mjör IA. Bjørndal L, et al. Quintessence Int. 2001 Oct;32(9):717-36. Quintessence Int. 2001. PMID: 11695140 Review. - [Response of odontoblastic and pulpal cells to carious lesions].
Farges JC, Joffre A, Magloire H. Farges JC, et al. C R Seances Soc Biol Fil. 1993;187(5):582-95. C R Seances Soc Biol Fil. 1993. PMID: 8069711 Review. French.
Cited by
- Understanding dental pulp inflammation: from signaling to structure.
Pohl S, Akamp T, Smeda M, Uderhardt S, Besold D, Krastl G, Galler KM, Buchalla W, Widbiller M. Pohl S, et al. Front Immunol. 2024 Oct 29;15:1474466. doi: 10.3389/fimmu.2024.1474466. eCollection 2024. Front Immunol. 2024. PMID: 39534600 Free PMC article. Review. - Applications and interventions of polymers and nanomaterials in alveolar bone regeneration and tooth dentistry.
Sharma P, Saurav S, Tabassum Z, Sood B, Kumar A, Malik T, Mohan A, Girdhar M. Sharma P, et al. RSC Adv. 2024 Nov 12;14(49):36226-36245. doi: 10.1039/d4ra06092j. eCollection 2024 Nov 11. RSC Adv. 2024. PMID: 39534053 Free PMC article. Review. - ANXA1 Enhances the Proangiogenic Potential of Human Dental Pulp Stem Cells.
Ma X, Zhao B, Wang C, Sun M, Dai Y, E L, Gao M, Liu X, Jia Y, Yue W, Liu H. Ma X, et al. Stem Cells Int. 2024 Oct 23;2024:7045341. doi: 10.1155/2024/7045341. eCollection 2024. Stem Cells Int. 2024. PMID: 39478978 Free PMC article. - Effect of polydatin on the viability and odontogenic differentiation of human dental pulp stem cells: An in-vitro study.
Al-Ateeq R, Elsafadi M, Al-Hadlaq S. Al-Ateeq R, et al. J Dent Sci. 2024 Oct;19(4):2332-2340. doi: 10.1016/j.jds.2024.02.005. Epub 2024 Feb 13. J Dent Sci. 2024. PMID: 39347037 Free PMC article. - Hypoxia-induced NLRP3 inflammasome activation via the HIF-1α/NF-κB signaling pathway in human dental pulp fibroblasts.
Wang D, Wang M, Sun S, Zhang C, Song Y, Li J, Song B, Lv H, Wang S, Jiang W. Wang D, et al. BMC Oral Health. 2024 Sep 29;24(1):1156. doi: 10.1186/s12903-024-04936-w. BMC Oral Health. 2024. PMID: 39343901 Free PMC article.
References
- Hamilton I. R. Ecological basis for dental caries. In: Kuramitsu H. K., Ellen R. P., editors. Oral Bacterial Ecology: The Molecular Basis. Wymondham, UK: Horizon Scientific Press; 2000. pp. 219–274.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical