Extreme low temperature tolerance in woody plants - PubMed (original) (raw)
Review
Extreme low temperature tolerance in woody plants
G Richard Strimbeck et al. Front Plant Sci. 2015.
Abstract
Woody plants in boreal to arctic environments and high mountains survive prolonged exposure to temperatures below -40°C and minimum temperatures below -60°C, and laboratory tests show that many of these species can also survive immersion in liquid nitrogen at -196°C. Studies of biochemical changes that occur during acclimation, including recent proteomic and metabolomic studies, have identified changes in carbohydrate and compatible solute concentrations, membrane lipid composition, and proteins, notably dehydrins, that may have important roles in survival at extreme low temperature (ELT). Consideration of the biophysical mechanisms of membrane stress and strain lead to the following hypotheses for cellular and molecular mechanisms of survival at ELT: (1) Changes in lipid composition stabilize membranes at temperatures above the lipid phase transition temperature (-20 to -30°C), preventing phase changes that result in irreversible injury. (2) High concentrations of oligosaccharides promote vitrification or high viscosity in the cytoplasm in freeze-dehydrated cells, which would prevent deleterious interactions between membranes. (3) Dehydrins bind membranes and further promote vitrification or act stearically to prevent membrane-membrane interactions.
Keywords: acclimation; biochemistry; cold; frost; hardening; hardiness; tolerance; vitirification.
Figures
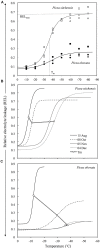
FIGURE 1
(A) Example of REL data and temperature response curves for fully acclimated needles from MLT and ELT tolerant species (Picea sitchensis and Picea obovata, respectively). Different symbols represent three different trees for each species. Horizontal and vertical dashed lines show location of the parameters RELmax and Tm, respectively for P. sitchensis (adapted from Strimbeck et al., 2007). (B,C) Changes in temperature response curves and Tm during acclimation for P. sitchensis (B) and P. obovata (C) (adapted from Strimbeck et al., 2008).
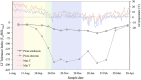
FIGURE 2
Daily maximum and minimum temperatures and seasonal acclimation and deacclimation in Picea sitchensis (an MLT tolerant species from a temperate oceanic environment) and P. obovata (an ELT tolerant Siberian species). Colored backgrounds indicate acclimation phases in P. obovata determined by cluster analysis of metabolomic data: pink, pre-acclimation; yellow, early acclimation; green, late acclimation; blue, fully acclimated (adapted from Strimbeck et al., 2008; Angelcheva et al., 2014).
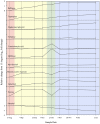
FIGURE 3
Relative concentrations of 11 metabolites during cold acclimation in Picea obovata. Colored backgrounds indicate acclimation phases determined by cluster analysis of metabolomic data: pink, pre-acclimation; yellow, early acclimation; green, late acclimation; blue, fully acclimated (adapted from Angelcheva et al., 2014).
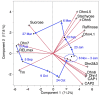
FIGURE 4
Principle component biplot of LT tolerance, sugar, and dehydrin (Dhn and CAP) data for P. obovata. Red lines indicate direction and strength of each variable. Tm and RELmax both decreased during acclimation, so greater low temperature tolerance was generally associated with higher levels of all sugars and dehydrins except sucrose and Dhn7. Dates and arrows indicate mean principle component scores for samples from three trees on each date. Data for 26 September and 24 April were excluded due to missing values for sugars and dehydrins, respectively (data from Strimbeck et al., 2008; Kjellsen et al., 2013).
Similar articles
- Metabolomic analysis of extreme freezing tolerance in Siberian spruce (Picea obovata).
Angelcheva L, Mishra Y, Antti H, Kjellsen TD, Funk C, Strimbeck RG, Schröder WP. Angelcheva L, et al. New Phytol. 2014 Nov;204(3):545-555. doi: 10.1111/nph.12950. Epub 2014 Aug 20. New Phytol. 2014. PMID: 25139797 - The role of abscisic acid and low temperature in chickpea (Cicer arietinum) cold tolerance. II. Effects on plasma membrane structure and function.
Bakht J, Bano A, Dominy P. Bakht J, et al. J Exp Bot. 2006;57(14):3707-15. doi: 10.1093/jxb/erl120. Epub 2006 Sep 21. J Exp Bot. 2006. PMID: 16990370 - Studies of Frost Hardiness in Woody Plants. II. Effect of Temperature on Hardening.
Sakai A. Sakai A. Plant Physiol. 1966 Feb;41(2):353-9. doi: 10.1104/pp.41.2.353. Plant Physiol. 1966. PMID: 16656262 Free PMC article. - Cold Hardiness in Trees: A Mini-Review.
Wisniewski M, Nassuth A, Arora R. Wisniewski M, et al. Front Plant Sci. 2018 Sep 20;9:1394. doi: 10.3389/fpls.2018.01394. eCollection 2018. Front Plant Sci. 2018. PMID: 30294340 Free PMC article. Review. - Molecular responses of plants to cold shock and cold acclimation.
Guy C. Guy C. J Mol Microbiol Biotechnol. 1999 Nov;1(2):231-42. J Mol Microbiol Biotechnol. 1999. PMID: 10943554 Review.
Cited by
- Standardization of electrolyte leakage data and a novel liquid nitrogen control improve measurements of cold hardiness in woody tissue.
Kovaleski AP, Grossman JJ. Kovaleski AP, et al. Plant Methods. 2021 May 22;17(1):53. doi: 10.1186/s13007-021-00755-0. Plant Methods. 2021. PMID: 34022929 Free PMC article. - The Roots of Plant Frost Hardiness and Tolerance.
Ambroise V, Legay S, Guerriero G, Hausman JF, Cuypers A, Sergeant K. Ambroise V, et al. Plant Cell Physiol. 2020 Jan 1;61(1):3-20. doi: 10.1093/pcp/pcz196. Plant Cell Physiol. 2020. PMID: 31626277 Free PMC article. Review. - Seasonal Metabolic Investigation in Pomegranate (Punica granatum L.) Highlights the Role of Amino Acids in Genotype- and Organ-Specific Adaptive Responses to Freezing Stress.
Yazdanpanah P, Jonoubi P, Zeinalabedini M, Rajaei H, Ghaffari MR, Vazifeshenas MR, Abdirad S. Yazdanpanah P, et al. Front Plant Sci. 2021 Aug 12;12:699139. doi: 10.3389/fpls.2021.699139. eCollection 2021. Front Plant Sci. 2021. PMID: 34456940 Free PMC article. - Transcriptomic changes reveal gene networks responding to the overexpression of a blueberry DWARF AND DELAYED FLOWERING 1 gene in transgenic blueberry plants.
Song GQ, Gao X. Song GQ, et al. BMC Plant Biol. 2017 Jun 19;17(1):106. doi: 10.1186/s12870-017-1053-z. BMC Plant Biol. 2017. PMID: 28629320 Free PMC article. - Transcriptional regulation of bark freezing tolerance in apple (Malus domestica Borkh.).
Liang Y, Wang S, Zhao C, Ma X, Zhao Y, Shao J, Li Y, Li H, Song H, Ma H, Li H, Zhang B, Zhang L. Liang Y, et al. Hortic Res. 2020 Dec 1;7(1):205. doi: 10.1038/s41438-020-00432-8. Hortic Res. 2020. PMID: 33328456 Free PMC article.
References
- Adams G. T. (1996). Wintertime Photostress in Red Spruce Foliage. Burlington, VT: University of Vermont.
- Anderson J. A., Kenna M. P., Taliaferro C. M. (1988). Cold hardiness of ‘Midiron’ and ’Tifgreen’ bermudagrass. Hortscience 23 748–750.
- Arora R., Rowland L. J. (2011). Physiological research on winter-hardiness: deacclimation resistance, reacclimation ability, photoprotection strategies, and a cold acclimation protocol design. Hortscience 46 1070–1078.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources