The point mutation UCH-L1 C152A protects primary neurons against cyclopentenone prostaglandin-induced cytotoxicity: implications for post-ischemic neuronal injury - PubMed (original) (raw)
The point mutation UCH-L1 C152A protects primary neurons against cyclopentenone prostaglandin-induced cytotoxicity: implications for post-ischemic neuronal injury
H Liu et al. Cell Death Dis. 2015.
Abstract
Cyclopentenone prostaglandins (CyPGs), such as 15-deoxy-Δ(12,14)-prostaglandin J2 (15dPGJ2), are reactive prostaglandin metabolites exerting a variety of biological effects. CyPGs are produced in ischemic brain and disrupt the ubiquitin-proteasome system (UPS). Ubiquitin-C-terminal hydrolase L1 (UCH-L1) is a brain-specific deubiquitinating enzyme that has been linked to neurodegenerative diseases. Using tandem mass spectrometry (MS) analyses, we found that the C152 site of UCH-L1 is adducted by CyPGs. Mutation of C152 to alanine (C152A) inhibited CyPG modification and conserved recombinant UCH-L1 protein hydrolase activity after 15dPGJ2 treatment. A knock-in (KI) mouse expressing the UCH-L1 C152A mutation was constructed with the bacterial artificial chromosome (BAC) technique. Brain expression and distribution of UCH-L1 in the KI mouse was similar to that of wild type (WT) as determined by western blotting. Primary cortical neurons derived from KI mice were resistant to 15dPGJ2 cytotoxicity compared with neurons from WT mice as detected by the WST-1 cell viability assay and caspase-3 and poly ADP ribose polymerase (PARP) cleavage. This protective effect was accompanied with significantly less ubiquitinated protein accumulation and aggregation as well as less UCH-L1 aggregation in C152A KI primary neurons after 15dPGJ2 treatment. Additionally, 15dPGJ2-induced axonal injury was also significantly attenuated in KI neurons as compared with WT. Taken together, these studies indicate that UCH-L1 function is important in hypoxic neuronal death, and the C152 site of UCH-L1 has a significant role in neuronal survival after hypoxic/ischemic injury.
Figures
Figure 1
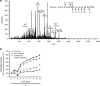
Adduct formation by 15dPGJ2 with cysteine152 is associated with decreased UCH-L1 hydrolase activity. (a) MS/MS spectrum of a tryptic fragment derived from Flag-tagged UCH-L1 expressed in primary neuronal cells following incubation with 15dPGJ2. Schematic representation of the amino-acid sequence, fragment ions, and the corresponding m/z values for the cysteine-modified tryptic peptide NEAIIQAAHDSVAQEGQC*R. 15dPGJ2 adduction (*) is shown to occur at C152. The spectrum was from an average of six tandem mass spectra from the doubly charged peptide ion at m/z 748.36 observed as eluted from C18 nanoLC separation with a retention range of 56.87–57.07 min. (b) Hydrolase activity in recombinant wild-type (WT) and mutant UCH-L1 C152A (C152A) proteins after incubation with 12.5 _μ_M 15dPGJ2 for 2 h measured at 0–12 min post substrate addition. Data are in arbitrary fluorescence units (AFUs) normalized to their respective time 0 and are expressed as means±S.E. _n_=2 per group. *P<0.05 between recombinant UCH-L1 C152A and WT 15dPGJ2-treated groups using repeated measures ANOVA with Bonferroni post hoc testing
Figure 2
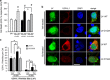
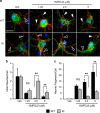
Overexpression of UCH-L1 C152A in rat primary neurons protects cells against 15dPGJ2-induced protein aggregation and cell death. Rat primary neurons were infected with flag-tagged lentivirus (LV)-UCH-L1 wild-type (WT) or LV-UCH-L1 C152A (C152A) at DIV2, then treated with 5 _μ_M 15dPGJ2 (15d) or vehicle (Veh) for 48 h at DIV10. (a) Cell death as measured by LDH release, normalized to respective vehicle control. _N_=6–12 per group. *_P_>0.05; #P<0.01 versus UN; Student's _t_-test. (b) Representative photos of LV-infected rat primary neurons after anti-flag immunocytochemistry (red, UCH-L1). Green is EGFP (indicating lentiviral infection) and blue is DAPI nuclear stain. Bar=10 _μ_m. Photos taken with an Olympus confocal microscope at 240 × . (c) UCH-L1 particle counts per cell: UCH-L1 particle sizes were measured and counted using NIH ImageJ software (National Institutes of Health). _n_=23–25 per group. *P<0.05 using repeated measures ANOVA with Bonferroni post hoc testing. (a and c) Data are means±S.E.
Figure 3
Generation of the UCH-L1 C152A knock-in (KI) mouse. (a) Schematic representation of homologous recombination of DNA fragments producing a point mutation in UCH-L1 converting the 152 cysteine to alanine. (b) UCH-L1 protein expression in UCH-L1 C152A KI and wild-type (WT) mouse brain cortex, hippocampus, and striatum. Brain regions (_n_=3 per group) were lysed and immunoblotted using anti-UCH-L1 and anti-GAPDH antibodies. Left: immunoblots; right: Graphical densitometric immunoblot analysis. (c) UCH-L1 protein expression in mouse UCH-L1 WT and KI primary neurons produced from UCH-L1 C152A KI and WT mice (_n_=4 per group). Left: immunoblots; right: Graphical densitometric immunoblot analysis. (b and c) Data are means±S.E. and normalized to their respective WT groups. GAPDH was used as a loading control
Figure 4
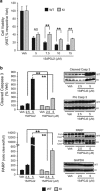
The UCH-L1 C152A mutation confers protection against 15dPGJ2-induced apoptotic cell death. (a and b) UCH-L1 C152A knock-in (KI) and wild-type (WT) primary neurons were treated with 15dPGJ2 or vehicle (DMSO, Veh) for 24 h. (a) Cell viability (WST-1 assay) after treatment with 5–15 _μ_M 15dPGJ2. _N_=6 per group. (b) Immunoblot detection of cleaved caspase 3 (Casp 3), pro Casp 3 (upper group), and PARP (full length: full, cleaved: clvd, lower group) using anti-caspase 3 and anti-PARP antibodies, respectively, after treatment with 2.5 or 5 _μ_M 15dPGJ2. GAPDH was used as a loading control. Left: Graphical densitometric analysis of immunoblots (right). _N_=3 per group. All: Data are means±S.E. and are normalized to their respective vehicle controls. **P<0.01 versus WT; NS, not significant. Black bar: WT; gray bar: UCH-L1 C152A
Figure 5
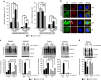
The UCH-L1 C152A mutation attenuates 15dPGJ2-induced protein aggregation in primary neurons. (a and b) Wild type (WT) and UCH-L1 C152A knock-in (KI) primary neurons were treated with 2.5 _μ_M 15dPGJ2 or vehicle (DMSO, Veh) for 24 h then immunostained with anti-ubiquitin (green) and anti-UCH-L1 (red) antibodies. (a) Ubiquitin (left) and UCH-L1 (right) particles were counted using ImageJ software (National Institutes of Health, _n_=23–25 cells per group). *P<0.05; **P<0.01 using one-way ANOVA with Bonferroni post hoc testing. (b) Representative fluoromicrographs of cells measured in (a). Blue is DAPI nuclear stain. Bar=10 _μ_m. Arrows indicate aggregates. (c) WT and KI cell lysates were prepared from WT and KI primary neurons treated with 15dPGJ2 or Vehicle (V) for 24 h and fractioned into RIPA-soluble and -insoluble fractions. (c) Representative immunoblots detecting poly-ubiquitin (left group) and Ubiquitin K48 (right group) in each fraction. (d) Immunoblot of ubiquitin K63 level in RIPA-soluble fraction. Corresponding densitometric immunoblot analysis is shown below. Data are normalized to their respective vehicle-treated groups. _β_-actin was used as a loading control. _N_=4 per group. *P<0.05; **P<0.01 versus WT. (a and c) Data are means±S.E.
Figure 6
The UCH-L1 C152A mutation protects 15dPGJ2-induced injury to neurites. Wild type (WT) and UCH-L1 C152A knock-in (KI) primary neurons were incubated with 1.25–5 _μ_M 15dPGJ2 or vehicle (Veh) for 24 h then immunostained with anti-NeuN (green) and anti-neurofilament L (red) antibodies. Blue is DAPI nuclear stain. (a) Representative confocal fluoromicrographs taken at 240 × . Bar=20 _μ_m. (b and c) Intact neurites (outlined arrows) and neurite fragments (solid arrows) were counted in eight fields per group and are normalized to the number of cells examined. Data are means±S.E. **P<0.01 versus WT; NS, not significant
Similar articles
- Modification of ubiquitin-C-terminal hydrolase-L1 by cyclopentenone prostaglandins exacerbates hypoxic injury.
Liu H, Li W, Ahmad M, Miller TM, Rose ME, Poloyac SM, Uechi G, Balasubramani M, Hickey RW, Graham SH. Liu H, et al. Neurobiol Dis. 2011 Feb;41(2):318-28. doi: 10.1016/j.nbd.2010.09.020. Epub 2010 Oct 13. Neurobiol Dis. 2011. PMID: 20933087 Free PMC article. - Role of UCHL1 in axonal injury and functional recovery after cerebral ischemia.
Liu H, Povysheva N, Rose ME, Mi Z, Banton JS, Li W, Chen F, Reay DP, Barrionuevo G, Zhang F, Graham SH. Liu H, et al. Proc Natl Acad Sci U S A. 2019 Mar 5;116(10):4643-4650. doi: 10.1073/pnas.1821282116. Epub 2019 Feb 13. Proc Natl Acad Sci U S A. 2019. PMID: 30760601 Free PMC article. - Increased generation of cyclopentenone prostaglandins after brain ischemia and their role in aggregation of ubiquitinated proteins in neurons.
Liu H, Li W, Ahmad M, Rose ME, Miller TM, Yu M, Chen J, Pascoe JL, Poloyac SM, Hickey RW, Graham SH. Liu H, et al. Neurotox Res. 2013 Aug;24(2):191-204. doi: 10.1007/s12640-013-9377-4. Epub 2013 Jan 25. Neurotox Res. 2013. PMID: 23355003 Free PMC article. - Ubiquitin C-terminal hydrolase L1 (UCH-L1): structure, distribution and roles in brain function and dysfunction.
Bishop P, Rocca D, Henley JM. Bishop P, et al. Biochem J. 2016 Aug 15;473(16):2453-62. doi: 10.1042/BCJ20160082. Biochem J. 2016. PMID: 27515257 Free PMC article. Review. - Role of the ubiquitin-proteasome system in brain ischemia: friend or foe?
Caldeira MV, Salazar IL, Curcio M, Canzoniero LM, Duarte CB. Caldeira MV, et al. Prog Neurobiol. 2014 Jan;112:50-69. doi: 10.1016/j.pneurobio.2013.10.003. Epub 2013 Oct 22. Prog Neurobiol. 2014. PMID: 24157661 Review.
Cited by
- In vivo transduction of neurons with TAT-UCH-L1 protects brain against controlled cortical impact injury.
Liu H, Rose ME, Ma X, Culver S, Dixon CE, Graham SH. Liu H, et al. PLoS One. 2017 May 24;12(5):e0178049. doi: 10.1371/journal.pone.0178049. eCollection 2017. PLoS One. 2017. PMID: 28542502 Free PMC article. - Novel therapies for combating chronic neuropathological sequelae of TBI.
Ikonomovic MD, Abrahamson EE, Carlson SW, Graham SH, Dixon CE. Ikonomovic MD, et al. Neuropharmacology. 2019 Feb;145(Pt B):160-176. doi: 10.1016/j.neuropharm.2018.06.021. Epub 2018 Jun 20. Neuropharmacology. 2019. PMID: 29933008 Free PMC article. Review. - Proteomic Analysis of Hippocampus in a Mouse Model of Depression Reveals Neuroprotective Function of Ubiquitin C-terminal Hydrolase L1 (UCH-L1) via Stress-induced Cysteine Oxidative Modifications.
Choi JE, Lee JJ, Kang W, Kim HJ, Cho JH, Han PL, Lee KJ. Choi JE, et al. Mol Cell Proteomics. 2018 Sep;17(9):1803-1823. doi: 10.1074/mcp.RA118.000835. Epub 2018 Jun 29. Mol Cell Proteomics. 2018. PMID: 29959188 Free PMC article. - Abolishing UCHL1's hydrolase activity exacerbates TBI-induced axonal injury and neuronal death in mice.
Mi Z, Liu H, Rose ME, Ma X, Reay DP, Ma J, Henchir J, Dixon CE, Graham SH. Mi Z, et al. Exp Neurol. 2021 Feb;336:113524. doi: 10.1016/j.expneurol.2020.113524. Epub 2020 Nov 4. Exp Neurol. 2021. PMID: 33159930 Free PMC article. - Abolishing UCHL1's hydrolase activity exacerbates ischemia-induced axonal injury and functional deficits in mice.
Mi Z, Povysheva N, Rose ME, Ma J, Zeh DJ, Harikumar N, Bhuiyan MIH, Graham SH. Mi Z, et al. J Cereb Blood Flow Metab. 2024 Nov;44(11):1349-1361. doi: 10.1177/0271678X241258809. Epub 2024 Jun 4. J Cereb Blood Flow Metab. 2024. PMID: 38833565 Free PMC article.
References
- 1Larsen CN, Price JS, Wilkinson KD. Substrate binding and catalysis by ubiquitin C-terminal hydrolases: identification of two active site residues. Biochemistry 1996; 35: 6735–6744. - PubMed
- 2Liu Y, Fallon L, Lashuel HA, Liu Z, Lansbury PT Jr. The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson's disease susceptibility. Cell 2002; 111: 209–218. - PubMed
- 3Sakurai M, Sekiguchi M, Zushida K, Yamada K, Nagamine S, Kabuta T et al. Reduction in memory in passive avoidance learning, exploratory behaviour and synaptic plasticity in mice with a spontaneous deletion in the ubiquitin C-terminal hydrolase L1 gene. Eur J Neurosci 2008; 27: 691–701. - PubMed
- 4Gong B, Cao Z, Zheng P, Vitolo OV, Liu S, Staniszewski A et al. Ubiquitin hydrolase Uch-L1 rescues beta-amyloid-induced decreases in synaptic function and contextual memory. Cell 2006; 126: 775–788. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000005/TR/NCATS NIH HHS/United States
- R01NS37549/NS/NINDS NIH HHS/United States
- P30 CA047904/CA/NCI NIH HHS/United States
- R01 NS037459/NS/NINDS NIH HHS/United States
- P30CA047904/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous