The LC Domain of hnRNPA2 Adopts Similar Conformations in Hydrogel Polymers, Liquid-like Droplets, and Nuclei - PubMed (original) (raw)
The LC Domain of hnRNPA2 Adopts Similar Conformations in Hydrogel Polymers, Liquid-like Droplets, and Nuclei
Siheng Xiang et al. Cell. 2015.
Abstract
Many DNA and RNA regulatory proteins contain polypeptide domains that are unstructured when analyzed in cell lysates. These domains are typified by an over-representation of a limited number of amino acids and have been termed prion-like, intrinsically disordered or low-complexity (LC) domains. When incubated at high concentration, certain of these LC domains polymerize into labile, amyloid-like fibers. Here, we report methods allowing the generation of a molecular footprint of the polymeric state of the LC domain of hnRNPA2. By deploying this footprinting technique to probe the structure of the native hnRNPA2 protein present in isolated nuclei, we offer evidence that its LC domain exists in a similar conformation as that described for recombinant polymers of the protein. These observations favor biologic utility to the polymerization of LC domains in the pathway of information transfer from gene to message to protein.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
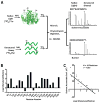
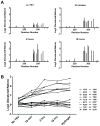
Figure 1. Differing Patterns of Acetylation of Folded and Denatured Samples of Glutathione-S-transferase Mediated by N-acetylimidazole
A. Folded glutathione-S-transferase (GST) was exposed to N-acetylimidazole (NAI) under conditions leading to roughly one modification per polypeptide chain, with the reaction quenched by the addition of 0.8 M Tris. A separate batch of GST grown in bacterial cells supplemented with 13C-labeled tyrosine was denatured in 5 M guanidine thiocyanate prior to NAI treatment. Following quenching with Tris, the two samples were mixed, digested with chymotrypsin and subjected to SILAC mass spectrometry. B. Nineteen acetylated side chains were scored for abundance in the two samples, yielding an NAI footprint. The degree of residue protection from NAI modification in the folded state, relative to the denatured state, is measured on the Y axis as log2 values. C. Plot showing the correlative relationship between the degree of protection from NAI in the folded state, relative to the denatured state (X-axis), and the measured level of solvent accessibility determined from the X-ray crystal structure of GST (Y-axis). See also Figure S1 and Table S1, S2.
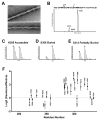
Figure 2. Footprint of NAI-mediated Acetylation of Recombinant hnRNPA2 Polymeric Fibers
A. Electron micrographs of negatively stained polymeric fibers formed from an mCherry:hnRNPA2 fusion protein (Experimental Procedures). Scale bar: 70 nm. B. HPLC separation of chymotryptic digestion products of the LC domain of hnRNPA2 corresponding to residues 302–319. The S312 acetylated peptide eluted earlier from the column than the S306 acetylated peptide, which – in turn – eluted earlier than the K305 acetylated peptide (Experimental Procedures). C. Relative abundances of the K305 acetylated peptides in folded versus denatured samples. D. Relative abundances of the S306 acetylated peptides in folded versus denatured samples. E. Relative abundances of the S312 acetylated peptides in folded versus denatured samples. F. NAI footprint of the LC domain of hnRNPA2 (All data are presented as means ± SD). See also Figure S2 and Table S3.
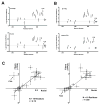
Figure 3. NAI Footprints of the LC Domain of hnRNPA2 Deduced from Recombinant Protein, Native Nuclear hnRNPA2, and Recombinant Protein Co-expressed with Peptidyl-prolyl Cis-trans Isomerase (PPIA)
A. NAI footprint of recombinant hnRNPA2 fibers as described in Figure 2 (upper footprint) compared with NAI footprint deduced from native, nuclear hnRNPA2 (lower footprint). Note that tyrosine 324 is protected from NAI modification in the folded form of hnRNPA2 in the recombinant form of hnRNPA2, but not in the footprint deduced from the native, nuclear protein. B. NAI footprint of recombinant hnRNPA2 co-expressed with active PPIA enzyme (upper footprint) compared with footprint of hnRNPA2 co-expressed with a catalytically inactive form of the enzyme (lower footprint). Note that co-expression of hnRNPA2 with the active form of PPIA causes tyrosine 324 to become exposed to NAI modification in the polymeric state. C. Plots showing the correlative relationship of the NAI footprint of recombinant hnRNPA2 to that of the native, nuclear form of the protein. Correlation plot on left compares the footprint of recombinant hnRNPA2 not exposed to the PPAI enzyme with the nuclear hnRNPA2 footprint. Correlation plot on right compares the footprint of recombinant hnRNPA2 co-expressed with the active PPIA enzyme with the nuclear hnRNPA2 footprint. See also Figure S3.
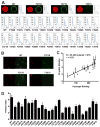
Figure 4. Correlative Relationship Between Binding of Mutated Variants of the LC Domain of hnRNPA2 to Hydrogels Relative to Their Partitioning into Liquid-like Droplets
A. All phenylalanine and tyrosine residues within the LC domain of hnRNPA2 were individually mutated to serine, expressed as GFP fusion proteins, purified and tested for binding to mCherry:hnRNPA2 hydrogel droplets (Experimental Procedures). Top figures show images of hydrogel binding by GFP linked to the native LC domain of hnRNPA2 (WT), the F215S mutant, the Y271S mutant and the F291S mutant. Confocal images were scanned to yield the signal intensity of bound GFP (Experimental Procedures), yielding the 26 scans in the lower part of the figure. X-axis indicates the scanned distance in μm, and Y-axis indicates the GFP signal intensity in arbitrary units. B. Liquid-like droplets formed upon binding of a PTB:hnRNPA2 fusion protein to a synthetic RNA containing five copies of the PTB recognition sequence (Experimental Procedures. See also Figure S4). The presence of a SNAP tag allowed the PTB:hnRNPA2 fusion protein to be appended with a red dye. When exposed to GFP alone, no partitioning into liquid-like droplets was observed (data not shown). When exposed to GFP fused to the native LC domain of hnRNPA2 (WT), clear evidence of partitioning was observed within minutes. Certain phenylalanine- or tyrosine-to-serine mutants partitioned well into liquid-like droplets (F215S), whereas others did not (Y271 and F291S). D. Partitioning into liquid-like droplets was quantified for all phenylalanine- and tyrosine-to-serine mutants that had been constructed and assayed for binding to mCherry:hnRNPA2 hydrogel droplets (A). Histogram shows relative levels of partitioning of GFP linked to the native (WT) LC domain of hnRNPA2 as compared with the 25 individual mutants. C. Plot showing the correlative relationship between hydrogel binding and partitioning into liquid-like droplets for GFP linked to the native (WT) LC domain of hnRNPA2 along with 25 individual phenylalanine- and tyrosine-to-serine mutants. See also Supplemental Data, Figure S2.
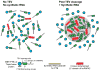
Figure 5. Liquid-like Droplets Display the Same NAI Footprint as Found in Hydrogel Polymers and the Native hnRNPA2 Present in Nuclei Freshly Isolated from Mammalian Cells
A. A fusion protein linking maltose binding protein (MBP) to the RNA binding domains of PTB and the LC domain of hnRNPA2 (Supplemental Data, Figure S4B) was co-expressed with the peptidyl-prolyl cis-trans isomerase enzyme (PPIA), purified, and mixed with a synthetic RNA containing five PTB binding sites. Addition of TEV protease triggered the rapid formation of liquid-like droplets (Figure 4B). Protein samples were footprinted with the NAI reagent as a function of time before and after TEV protease cleavage. Hints of the NAI footprint could be seen in the protein sample before exposure to TEV protease, and the intensity of the footprint was sequentially enhanced at the 10 minute and 2 and 18 hours post-cleavage time points. B. The log2 ratio of NAI protection for all of the 18 acetylated amino acids is plotted on the Y-axis as a function of time post-exposure to TEV protease (X-axis). See also Supplemental Data Table S3.
Figure 6. Graphical Representation of Conversion of Soluble MBP:PTB:hnRNP LC Fusion Protein into Liquid-like Droplet State
The triple fusion linking maltose binding protein (MBP = blue circle), the RNA binding domain of pyrimidine track binding protein (PTB = green rectangle), and the low complexity domain of hnRNPA2 (LC domain = wavy line) remains soluble and partially polymerized via the LC domain (red sheets) prior to TEV cleavage and exposure to synthetic RNA containing five PTB binding sites (yellow rectangle). Following TEV cleavage and exposure to RNA, MBP is left in solution and PTB:hnRNP LC domain fusion protein partitions into liquid-like droplet (grey shading) in a state of enhanced polymerization.
Similar articles
- The SH3 domain of Fyn kinase interacts with and induces liquid-liquid phase separation of the low-complexity domain of hnRNPA2.
Amaya J, Ryan VH, Fawzi NL. Amaya J, et al. J Biol Chem. 2018 Dec 21;293(51):19522-19531. doi: 10.1074/jbc.RA118.005120. Epub 2018 Nov 5. J Biol Chem. 2018. PMID: 30397184 Free PMC article. - Tyrosine phosphorylation regulates hnRNPA2 granule protein partitioning and reduces neurodegeneration.
Ryan VH, Perdikari TM, Naik MT, Saueressig CF, Lins J, Dignon GL, Mittal J, Hart AC, Fawzi NL. Ryan VH, et al. EMBO J. 2021 Feb 1;40(3):e105001. doi: 10.15252/embj.2020105001. Epub 2020 Dec 22. EMBO J. 2021. PMID: 33349959 Free PMC article. - Structural characterization of the D290V mutation site in hnRNPA2 low-complexity-domain polymers.
Murray DT, Zhou X, Kato M, Xiang S, Tycko R, McKnight SL. Murray DT, et al. Proc Natl Acad Sci U S A. 2018 Oct 16;115(42):E9782-E9791. doi: 10.1073/pnas.1806174115. Epub 2018 Oct 2. Proc Natl Acad Sci U S A. 2018. PMID: 30279180 Free PMC article. - Cross-β Polymerization of Low Complexity Sequence Domains.
Kato M, McKnight SL. Kato M, et al. Cold Spring Harb Perspect Biol. 2017 Mar 1;9(3):a023598. doi: 10.1101/cshperspect.a023598. Cold Spring Harb Perspect Biol. 2017. PMID: 27836835 Free PMC article. Review. - Redox-mediated regulation of low complexity domain self-association.
Kato M, Tu BP, McKnight SL. Kato M, et al. Curr Opin Genet Dev. 2021 Apr;67:111-118. doi: 10.1016/j.gde.2020.12.006. Epub 2021 Jan 14. Curr Opin Genet Dev. 2021. PMID: 33454579 Free PMC article. Review.
Cited by
- Mechanisms and regulation underlying membraneless organelle plasticity control.
Ismail H, Liu X, Yang F, Li J, Zahid A, Dou Z, Liu X, Yao X. Ismail H, et al. J Mol Cell Biol. 2021 Aug 4;13(4):239-258. doi: 10.1093/jmcb/mjab028. J Mol Cell Biol. 2021. PMID: 33914074 Free PMC article. Review. - The Structure and Dynamics of Higher-Order Assemblies: Amyloids, Signalosomes, and Granules.
Wu H, Fuxreiter M. Wu H, et al. Cell. 2016 May 19;165(5):1055-1066. doi: 10.1016/j.cell.2016.05.004. Cell. 2016. PMID: 27203110 Free PMC article. Review. - F/YGG-motif is an intrinsically disordered nucleic-acid binding motif.
Van Lindt J, Lazar T, Pakravan D, Demulder M, Meszaros A, Van Den Bosch L, Maes D, Tompa P. Van Lindt J, et al. RNA Biol. 2022;19(1):622-635. doi: 10.1080/15476286.2022.2066336. Epub 2021 Dec 31. RNA Biol. 2022. PMID: 35491929 Free PMC article. - Quantifying Dynamics in Phase-Separated Condensates Using Fluorescence Recovery after Photobleaching.
Taylor NO, Wei MT, Stone HA, Brangwynne CP. Taylor NO, et al. Biophys J. 2019 Oct 1;117(7):1285-1300. doi: 10.1016/j.bpj.2019.08.030. Epub 2019 Aug 30. Biophys J. 2019. PMID: 31540706 Free PMC article. - New Insights into Dyskerin-CypA Interaction: Implications for X-Linked Dyskeratosis Congenita and Beyond.
Belli V, Maiello D, Di Lorenzo C, Furia M, Vicidomini R, Turano M. Belli V, et al. Genes (Basel). 2023 Sep 6;14(9):1766. doi: 10.3390/genes14091766. Genes (Basel). 2023. PMID: 37761906 Free PMC article.
References
- Anderson P, Kedersha N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nature reviews Molecular cell biology. 2009;10:430–436. - PubMed
- Astbury WT, Beighton E, Parker KD. The cross-beta configuration in supercontracted proteins. Biochimica et biophysica acta. 1959;35:17–25. - PubMed
- Baxa U, Wickner RB, Steven AC, Anderson DE, Marekov LN, Yau WM, Tycko R. Characterization of beta-sheet structure in Ure2p1-89 yeast prion fibrils by solid-state nuclear magnetic resonance. Biochemistry. 2007;46:13149–13162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases