DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation - PubMed (original) (raw)
Review
DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation
Melody G Duvall et al. Eur J Pharmacol. 2016.
Abstract
Essential fatty acids can serve as important regulators of inflammation. A new window into mechanisms for the resolution of inflammation was opened with the identification and structural elucidation of mediators derived from these fatty acids with pro-resolving capacity. Inflammation is necessary to ensure the continued health of the organism after an insult or injury; however, unrestrained inflammation can lead to injury "from within" and chronic changes that may prove both morbid and fatal. The resolution phase of inflammation, once thought to be a passive event, is now known to be a highly regulated, active, and complex program that terminates the inflammatory response once the threat has been contained. Specialized pro-resolving mediators (SPMs) are biosynthesized from omega-3 essential fatty acids to resolvins, protectins, and maresins and from omega-6 fatty acids to lipoxins. Through cell-specific actions mediated through select receptors, these SPMs are potent regulators of neutrophil infiltration, cytokine and chemokine production, and clearance of apoptotic neutrophils by macrophages, promoting a return to tissue homeostasis. This process appears to be defective in several common human lung diseases, such as asthma and COPD, which are characterized by chronic unrestrained inflammation and significant associated morbidity. Here, we highlight translational research in animal models of disease and with human subjects that sheds light on this rapidly evolving area of science and review the molecular and cellular components of the resolution of lung inflammation.
Keywords: Fatty acids; Inflammation; Resolution; lung.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures
Fig. 1
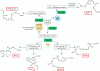
EPA and DHA are available at sites of acute inflammation. A) The omega-3 fatty acids EPA and DHA circulate in plasma. On injury they move with edema into the tissue sites of acute inflammation where they are converted to exudate SPMs to interact with local immune cells. Shown is a cartoon representation of acute lung inflammation in the setting of allergic asthma. B) DHA and EPA can also be released from the cell membranes through the actions of secretory phospholipase A2 (sPLA2). C) DHA (as LPC) is transported across the blood brain barrier via the transporter Mfsd2a.
Fig. 2
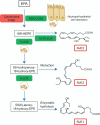
Biosynthesis of D-series resolvins, maresins, and protectins. Docosahexaenoic acid (DHA; C22:6n-3; grey box) is converted by 15-LOX to the intermediate 17S-hydroperoxy-DHA which is then further converted by neutrophil derived 5-LOX to the D-series resolvins, RvD1 through RvD4. DHA can also be converted via 15-LOX into a 16, 17-epoxy-protectin intermediate and then onto to protectin D1. DHA is converted via 12-LOX in macrophages to a 13S, 14S-epoxy-maresin and then through enzymatic hydrolysis to MaR1.
Fig. 3
Biosynthesis of E-series resolvins. Eicosapentaenoic acid (EPA; C20:5n-3; grey box) is converted via aspirin acetylated COX-2 or by cytochrome p450 in microbes to the intermediate 18R-hydroperoxy-EPE (18R-HEPE). The intermediate 18R-HEPE can be further transformed via neutrophil 5-LOX to an intermediate for subsequent enzymatic conversion to RvE1 or via reduction to RvE2. RvE3 is generated directly from 18R-HEPE via 12/15-LOX.
Fig. 4
Cellular targets and receptors for resolvins, protectins, and maresins. SPMs signal through specific 7-transmembrane G-protein coupled receptors. Lipoxin A4 (LXA4) and the D-series resolvins, in particular RvD1 are agonists for the ALX/FPR2 receptor that is present on a wide variety of leukocytes and other cells. RvE1 and RvE2 are agonists for the CMKLR1/ERV receptor, which is widely expressed on immune cells, and antagonists at the BLT1 receptor. The D-series resolvins are also agonists at the DRV1 and DRV2 receptors. The receptors for protectins and maresins have yet to be molecularly characterized. SPMs signal through these specific receptors to exert influence on leukocytes including halting neutrophil trafficking, promoting macrophage phagocytosis of apoptotic cells and microbes, and blocking further pro-inflammatory cytokine and chemokine production.
Similar articles
- EPA- and DHA-derived resolvins' actions in inflammatory bowel disease.
Schwanke RC, Marcon R, Bento AF, Calixto JB. Schwanke RC, et al. Eur J Pharmacol. 2016 Aug 15;785:156-164. doi: 10.1016/j.ejphar.2015.08.050. Epub 2015 Aug 29. Eur J Pharmacol. 2016. PMID: 26325092 Review. - Pro-resolving mediators produced from EPA and DHA: Overview of the pathways involved and their mechanisms in metabolic syndrome and related liver diseases.
López-Vicario C, Rius B, Alcaraz-Quiles J, García-Alonso V, Lopategi A, Titos E, Clària J. López-Vicario C, et al. Eur J Pharmacol. 2016 Aug 15;785:133-143. doi: 10.1016/j.ejphar.2015.03.092. Epub 2015 May 15. Eur J Pharmacol. 2016. PMID: 25987424 Review. - Immuno-Resolving Ability of Resolvins, Protectins, and Maresins Derived from Omega-3 Fatty Acids in Metabolic Syndrome.
Kwon Y. Kwon Y. Mol Nutr Food Res. 2020 Feb;64(4):e1900824. doi: 10.1002/mnfr.201900824. Epub 2019 Dec 15. Mol Nutr Food Res. 2020. PMID: 31797565 Review.
Cited by
- Exploration of n-6 and n-3 Polyunsaturated Fatty Acids Metabolites Associated with Nutritional Levels in Patients with Severe Stable Chronic Obstructive Pulmonary Disease.
Xue M, Cai C, Guan L, Xu Y, Lin J, Zeng Y, Hu H, Chen R, Wang H, Zhou L, Sun B. Xue M, et al. Int J Chron Obstruct Pulmon Dis. 2020 Jul 10;15:1633-1642. doi: 10.2147/COPD.S245617. eCollection 2020. Int J Chron Obstruct Pulmon Dis. 2020. PMID: 32764909 Free PMC article. - Regulation of Tissue Inflammation by 12-Lipoxygenases.
Kulkarni A, Nadler JL, Mirmira RG, Casimiro I. Kulkarni A, et al. Biomolecules. 2021 May 11;11(5):717. doi: 10.3390/biom11050717. Biomolecules. 2021. PMID: 34064822 Free PMC article. Review. - Nutraceuticals for Complementary Treatment of Multisystem Inflammatory Syndrome in Children: A Perspective from Their Use in COVID-19.
Estrada-Luna D, Carreón-Torres E, González-Reyes S, Martínez-Salazar MF, Ortiz-Rodríguez MA, Ramírez-Moreno E, Arias-Rico J, Jiménez-Osorio AS. Estrada-Luna D, et al. Life (Basel). 2022 Oct 20;12(10):1652. doi: 10.3390/life12101652. Life (Basel). 2022. PMID: 36295088 Free PMC article. Review. - A specific combined long-chain polyunsaturated fatty acid supplementation reverses fatty acid profile alterations in a mouse model of chronic asthma.
Fussbroich D, Zimmermann K, Göpel A, Eickmeier O, Trischler J, Zielen S, Schubert R, Beermann C. Fussbroich D, et al. Lipids Health Dis. 2019 Jan 18;18(1):16. doi: 10.1186/s12944-018-0947-6. Lipids Health Dis. 2019. PMID: 30658644 Free PMC article. - Association between Polyunsaturated Fatty Acid Profile and Bronchial Inflammation in Bronchiolitis Obliterans.
Jerkic SP, Bächle L, Duecker RP, Gronau L, Chiocchetti AG, Zielen S, Schubert R. Jerkic SP, et al. Mediators Inflamm. 2023 Jul 5;2023:3406399. doi: 10.1155/2023/3406399. eCollection 2023. Mediators Inflamm. 2023. PMID: 37448886 Free PMC article.
References
- Abdulnour RE, Dalli J, Colby JK, Krishnamoorthy N, Timmons JY, Tan SH, Colas RA, Petasis NA, Serhan CN, Levy BD. Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:16526–16531. - PMC - PubMed
- Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–1118. - PubMed
- Aoki H, Hisada T, Ishizuka T, Utsugi M, Kawata T, Shimizu Y, Okajima F, Dobashi K, Mori M. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochemical and biophysical research communications. 2008;367:509–515. - PubMed
- Ariel A, Li PL, Wang W, Tang WX, Fredman G, Hong S, Gotlinger KH, Serhan CN. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. The Journal of biological chemistry. 2005;280:43079–43086. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 HL108712/HL/NHLBI NIH HHS/United States
- P01 GM095467/GM/NIGMS NIH HHS/United States
- R01 HL122531/HL/NHLBI NIH HHS/United States
- U10 HL109172/HL/NHLBI NIH HHS/United States
- U24 AI118656/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials