Sigma-1 receptor mediates cocaine-induced transcriptional regulation by recruiting chromatin-remodeling factors at the nuclear envelope - PubMed (original) (raw)
Sigma-1 receptor mediates cocaine-induced transcriptional regulation by recruiting chromatin-remodeling factors at the nuclear envelope
Shang-Yi A Tsai et al. Proc Natl Acad Sci U S A. 2015.
Abstract
The sigma-1 receptor (Sig-1R) chaperone at the endoplasmic reticulum (ER) plays important roles in cellular regulation. Here we found a new function of Sig-1R, in that it translocates from the ER to the nuclear envelope (NE) to recruit chromatin-remodeling molecules and regulate the gene transcription thereof. Sig-1Rs mainly reside at the ER-mitochondrion interface. However, on stimulation by agonists such as cocaine, Sig-1Rs translocate from ER to the NE, where Sig-1Rs bind NE protein emerin and recruit chromatin-remodeling molecules, including lamin A/C, barrier-to-autointegration factor (BAF), and histone deacetylase (HDAC), to form a complex with the gene repressor specific protein 3 (Sp3). Knockdown of Sig-1Rs attenuates the complex formation. Cocaine was found to suppress the gene expression of monoamine oxidase B (MAOB) in the brain of wild-type but not Sig-1R knockout mouse. A single dose of cocaine (20 mg/kg) in rats suppresses the level of MAOB at nuclear accumbens without affecting the level of dopamine transporter. Daily injections of cocaine in rats caused behavioral sensitization. Withdrawal from cocaine in cocaine-sensitized rats induced an apparent time-dependent rebound of the MAOB protein level to about 200% over control on day 14 after withdrawal. Treatment of cocaine-withdrawn rats with the MAOB inhibitor deprenyl completely alleviated the behavioral sensitization to cocaine. Our results demonstrate a role of Sig-1R in transcriptional regulation and suggest cocaine may work through this newly discovered genomic action to achieve its addictive action. Results also suggest the MAOB inhibitor deprenyl as a therapeutic agent to block certain actions of cocaine during withdrawal.
Keywords: MAOB; cocaine; deprenyl; emerin; sigma-1 receptor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
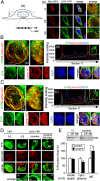
Fig. 1.
Sig-1R targets the NE in cocaine-treated cells. (A, a) Diagram illustrates the confocal laser scanning of individual planes (–3) of the nucleus. (A, b) Colocalization of endogenous Sig-1R with nuclear pore complex (on NE) marker RanBP2 in NG108 cells. Sig-1R, green; RanBP2, red; DNA, blue. (B and C) The whole-nucleus image analysis of Sig-1R-YFP and lamin A/C in differentiated Neuro2A cells under basal condition (B) or cocaine (5 μM) treatment for 1 h (C). In B, a and C, a, left panels are the top-down view (_z_-axis), and right panels are a side view (_x_-axis) of the 3D reconstruction of images. (B, b and C, b) Intensity scaling of a comparable section (section 11) taken from SI Appendix, Fig. S1 B and C, respectively. Note the increase of relative intensity at the NE in cocaine-treated cells. (D) Sig-1R localization on NE in NG108 cells is intensified by cocaine: the effect of cocaine (5 µM, 1 h) is blocked by the Sig-1R antagonist BD1063 (100 nM). (E) Fractionation study on the existence of Sig-1Rs at the nuclear membrane. Neuro2A cells were treated with saline (−) or cocaine for 1 h. Cellular homogenates were prepared for the fractionation assay as described in SI Appendix, Materials and Methods. n = 3 independent experiments (t test; *P < 0.05).
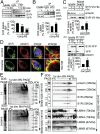
Fig. 2.
Sig-1R interacts with the NE protein emerin, and cocaine intensifies the interaction. (A) Cocaine causes the dissociation of Sig-1R from BiP in a dose-dependent manner. Sig-1R-YFP-expressing Neuro2A cells were treated with saline or different doses of cocaine (0, 1, 5, 20 μM) for 1 h. Cell lysates were immunoprecipitated with anti-GFP/YFP antibodies followed by immunoblot. Bars represent means ± SEM from three independent experiments (t test: **P < 0.01; ***P < 0.001). (B) Sig-1R association with lamin A/C is intensified by cocaine. The IP was with anti-lamin A/C antibodies. Bars represent means ± SEM from three independent experiments (t test: **P < 0.01). (C) Cocaine intensifies the Sig-1R-emerin interaction. Sig-1R-V5-His-expressing (a) and YFP- or Sig-1R-YFP-expressing (b) Neuro2A cells were treated with saline or cocaine (5 μM) for 1 h before extraction and assayed respectively in the IP and blotting. Bars represent means ± SEM from three independent experiments (t test: **P < 0.01). (D) Confocal images of the colocalization of Sig-1R and emerin in NG-108 cells in the absence or presence of cocaine (5 μM, 1 h). (E and F) Protein complex determination by the native blue gel method. (E) Neuro2A cells were treated with saline or cocaine (5 µM) for 1 h. Cell lysates were subjected to 1D SDS/PAGE (Left) or 2D BN-PAGE/SDS/PAGE (Right). Coomassie blue staining is shown. (F) Immunoblot assay after the 1D SDS/PAGE (Left) or the 2D BN-PAGE/SDS/PAGE (Right) by using respective antibodies to identify and analyze for components of the protein complex. Cocaine did not change the pattern of emerin, but apparently increased the complex formation among emerin, Sig-1R, and lamin A/C.
Fig. 3.
Sig-1R recruits the emerin/BAF/HDAC complex: cocaine intensifies the complex formation in a Sig-1R-dependent manner. (A_–_D) Sig-1R interaction with HDAC1, HDAC2, HDAC3: cocaine increases the interaction. (A) Endogenous Sig-1R interaction with HDAC1 and HDAC2 in Neuro2A cells. (B) Cocaine increases the interaction between Sig-1R and HDACs. Cocaine’s (5 μM, 1 h) effects on the association between endogenous Sig-1R and HDAC1 and HDAC2 (a) and between Sig-1R-V5-His and HDAC3 (b). (C and D) Sig-1R forms a complex with HDACs, BAF, and emerin: cocaine (5 μM, 1 h) increases the complex formation. (C) Endogenous Sig-1R binds HDAC1, HDAC2, BAF, and emerin; Bip is also shown. Bar represents means ± SEM from three to five independent experiments. Cocaine, as expected, decreases the interaction between Sig-1R and BiP (t test: *P < 0.05; **P < 0.01). (D) Time-dependent formation of the complex induced by cocaine (5 μM). (E and F) Cocaine enhances the interaction between endogenous Sig-1R and indicated proteins in NG108 cells (E) and in the prefrontal cortex of mouse brain (16 h after a 20 mg/kg cocaine injection, i.p.) (F). (G) Cocaine-increased interaction between emerin and HDAC2 is blocked by the knockdown of Sig-1Rs. Cells were transfected with adeno-associated virus (AAV)-shSig-1R or control AAV vector for 48 h before treating with saline or cocaine (5 μM) for 1 h. Bar represents means ± range from two independent experiments.
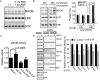
Fig. 4.
Sig-1R knockdown increases MAOB protein expression while reducing the binding of the Sig-1R/emerin/BAF/HDAC/specific protein 3 (Sp3) complex to the promoter of MAOB. (A) Presence of MAOB in differentiated Neuro2A cells after 2 d of the induction of differentiation. ERK, loading control. (B) Increase of MAOB levels in Sig-1R knockdown cells 24 or 48 h after transfection with the AAV-shSig-1R or control AAV vector. (Right) Results from three independent experiments (bars = means ± SEM normalized to the ERK loading control; t test: **P < 0.01). (C) Enhanced enzymatic activity of MAOB in Sig-1R knockdown primary neurons. Primary cultured E14 mouse cortical neurons expressing Sig-1R siRNA (siSig-1R) exhibited higher MAOB enzyme activity compared with neurons expressing control siRNA (siCon; t test: **P < 0.01; means ± SEM; n = 3). R-(−)-deprenyl hydrochloride (DP) reduced MAOB activity in both siCon and siSig-1R neurons. (D) Reduction of the complex protein binding to the promoter of MAOB, as seen in the DAPA. Neuro2A cells were transfected with AAV-shSig-1R or control AAV vector for 48 h before assay. The lysates and the products of DAPA were analyzed with immunoblotting by using antibodies as indicated. Beads without DNA probe serves as the negative control. (Right) Results from the DNA binding activity of those factors from immunoblotting were quantified and presented as the means ± SEM from three independent experiments (t test: *P < 0.05; **P < 0.01; ***P < 0.001).
Fig. 5.
Cocaine effects on both the MAOB expression and the binding of the Sig-1R-recruited protein complex to the promoter of MAOB. (A) Cocaine dose-dependently decreased MAOB protein levels in Neuro2A cells. (B, a) Cocaine dose-dependently and time-dependently increased HDAC1 and HDAC2 binding to the MAOB promoter: DAPA assays. (B, b) Quantified results from B, a. Bar = means ± SEM (n = 3 independent experiments (HDAC1 t test: *P < 0.05; **P < 0.01; ***P < 0.001; HDAC2 t test: #P < 0.05; #P < 0.01). (C) Cocaine (5 µM, 1h) increased the binding of Sig-1R-recruited proteins to the MAOB promoter in mouse primary cortical neurons. (D, a) Scheme of the chromatin IP analysis. (D, b) DNA analysis from chromatin IP of the in vivo effect of cocaine on the binding of Sig-1R-recruited proteins to the MAOB promoter in Neuro2A cells. (D, c) Quantified results from D, b. Bar = means ± SEM of signals of the PCR products from chromatin IP normalized to the total DNA (t test: **P < 0.01; ***P < 0.001; n = 3). (E–H) Ex vivo (E) and in vivo (F–H) examinations of cocaine effects on the MAOB protein levels. (E) Primary mouse cortical neurons treated with different doses of cocaine for 18 h before assay. (F) Mouse received 20 mg/kg cocaine, and 16 h later the prefrontal cortex of mouse was processed for Western blotting. Bar represents means ± SEM (t test: **P < 0.01; n = 3 independent experiments). (G and H) Tissues from prefrontal cortex (G) and nucleus accumbens (H) of rats were obtained at different points after administration of cocaine (20 mg/kg). Bar represents means ± SEM or range from three (G, t test: *P < 0.05; **P < 0.01) or two (H) independent experiments, respectively.
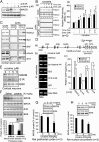
Fig. 6.
Sig-1R-dependency of cocaine effects on the formation of the recruited complex and the expression of the MAOB protein. (A) Reduced binding of recruited proteins to the MAOB promoter (DAPA assay) in Sig-1R-knockdown NG108 cells and the inability of cocaine (5 μM, 1 h) to enhance the promoter binding of those proteins in those cells. (B) Bar represents means ± SEM from three independent experiments from A (t test: *P < 0.05; **P < 0.01; ***P < 0.001). COC, cocaine. (C and D) Effects of cocaine (20 mg/kg; 16 h) on the MAOB, MAOA, and DAT levels in wild-type or Sig-1R knockout mice. Prefrontal cortices were examined. Bar represents means ± SEM in D (t test: **P < 0.01; n = 3 independent experiments from C). (E) Exogenous expression of Sig-1Rs in primary neurons from Sig-1R knockout mouse restores cocaine’s (1 µM, 24 h) ability to suppress MAOB expression. Neurons were transduced with either AAV-GFP controls (lanes 1 and 2) or AAV-Sig-1R (lanes 3 and 4). Bar represents means ± SEM (t test: *P < 0.05; n = 3 independent experiments).
Fig. 7.
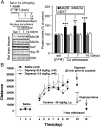
Cocaine withdrawal increases MAOB at the nucleus accumbens: the MAOB inhibitor deprenyl blocks cocaine’s action in behaviorally sensitized rats. Rats received daily injections of cocaine (20 mg/kg of cocaine, i.p.) for 7 d and were examined for locomotion daily. Rats then were withdrawn from cocaine for 1, 7, and 14 d. On day 7 after withdrawal, rats received deprenyl (0.5 mg/kg or 1.0 mg/kg, i.p.) 30 min before test for cocaine-induced locomotion. (A) MAOB, MAOA, and DAT protein levels at the nucleus accumbens during withdrawal from cocaine. Bars represent means ± SEM (n = 4 independent experiments from four rats; t test, **P < 0.01; ***P < 0.001). (B) Repeated cocaine injections produced significant locomotor sensitization in all three groups of rats before deprenyl pretreatment. On the test day (day 18; i.e., 7 d after withdrawal), pretreatment with deprenyl (0.5, 1.0 mg/kg, i.p.) dose-dependently blocked cocaine-induced increase in locomotion and locomotor sensitization (_F_2,21 = 6.74, P < 0.01, one-way ANOVA; ***P < 0.001, t test). Deprenyl alone (0.5, 1.0 mg/kg, i.p.), given a day after the cocaine blockade test, did not affect the locomotion.
Similar articles
- Genomic Action of Sigma-1 Receptor Chaperone Relates to Neuropathic Pain.
Wang SM, Goguadze N, Kimura Y, Yasui Y, Pan B, Wang TY, Nakamura Y, Lin YT, Hogan QH, Wilson KL, Su TP, Wu HE. Wang SM, et al. Mol Neurobiol. 2021 Jun;58(6):2523-2541. doi: 10.1007/s12035-020-02276-8. Epub 2021 Jan 18. Mol Neurobiol. 2021. PMID: 33459966 Free PMC article. - Dynamic interaction between sigma-1 receptor and Kv1.2 shapes neuronal and behavioral responses to cocaine.
Kourrich S, Hayashi T, Chuang JY, Tsai SY, Su TP, Bonci A. Kourrich S, et al. Cell. 2013 Jan 17;152(1-2):236-47. doi: 10.1016/j.cell.2012.12.004. Cell. 2013. PMID: 23332758 Free PMC article. - Protein kinase A activation down-regulates, whereas extracellular signal-regulated kinase activation up-regulates sigma-1 receptors in B-104 cells: Implication for neuroplasticity.
Cormaci G, Mori T, Hayashi T, Su TP. Cormaci G, et al. J Pharmacol Exp Ther. 2007 Jan;320(1):202-10. doi: 10.1124/jpet.106.108415. Epub 2006 Oct 18. J Pharmacol Exp Ther. 2007. PMID: 17050780 - The Sigma-1 Receptor as a Pluripotent Modulator in Living Systems.
Su TP, Su TC, Nakamura Y, Tsai SY. Su TP, et al. Trends Pharmacol Sci. 2016 Apr;37(4):262-278. doi: 10.1016/j.tips.2016.01.003. Epub 2016 Feb 9. Trends Pharmacol Sci. 2016. PMID: 26869505 Free PMC article. Review. - The sigma-1 receptor: roles in neuronal plasticity and disease.
Kourrich S, Su TP, Fujimoto M, Bonci A. Kourrich S, et al. Trends Neurosci. 2012 Dec;35(12):762-71. doi: 10.1016/j.tins.2012.09.007. Epub 2012 Oct 23. Trends Neurosci. 2012. PMID: 23102998 Free PMC article. Review.
Cited by
- Epigenetic mechanisms of rapid-acting antidepressants.
Inserra A, Campanale A, Rezai T, Romualdi P, Rubino T. Inserra A, et al. Transl Psychiatry. 2024 Sep 4;14(1):359. doi: 10.1038/s41398-024-03055-y. Transl Psychiatry. 2024. PMID: 39231927 Free PMC article. Review. - A high-affinity cocaine binding site associated with the brain acid soluble protein 1.
Harraz MM, Malla AP, Semenza ER, Shishikura M, Singh M, Hwang Y, Kang IG, Song YJ, Snowman AM, Cortés P, Karuppagounder SS, Dawson TM, Dawson VL, Snyder SH. Harraz MM, et al. Proc Natl Acad Sci U S A. 2022 Apr 19;119(16):e2200545119. doi: 10.1073/pnas.2200545119. Epub 2022 Apr 11. Proc Natl Acad Sci U S A. 2022. PMID: 35412917 Free PMC article. - Cocaine-induced endocannabinoid signaling mediated by sigma-1 receptors and extracellular vesicle secretion.
Nakamura Y, Dryanovski DI, Kimura Y, Jackson SN, Woods AS, Yasui Y, Tsai SY, Patel S, Covey DP, Su TP, Lupica CR. Nakamura Y, et al. Elife. 2019 Oct 9;8:e47209. doi: 10.7554/eLife.47209. Elife. 2019. PMID: 31596232 Free PMC article. - Loss of Sigma-1 Receptor Chaperone Promotes Astrocytosis and Enhances the Nrf2 Antioxidant Defense.
Weng TY, Hung DT, Su TP, Tsai SA. Weng TY, et al. Oxid Med Cell Longev. 2017;2017:4582135. doi: 10.1155/2017/4582135. Epub 2017 Aug 14. Oxid Med Cell Longev. 2017. PMID: 28883901 Free PMC article. - Sigma 1 Receptor and Its Pivotal Role in Neurological Disorders.
Shokr MM, Badawi GA, Elshazly SM, Zaki HF, Mohamed AF. Shokr MM, et al. ACS Pharmacol Transl Sci. 2024 Dec 30;8(1):47-65. doi: 10.1021/acsptsci.4c00564. eCollection 2025 Jan 10. ACS Pharmacol Transl Sci. 2024. PMID: 39816800 Review.
References
- de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456(7222):605–610. - PubMed
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131(3):596–610. - PubMed
- Boehning D, et al. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5(12):1051–1061. - PubMed
- Su TP, London ED, Jaffe JH. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science. 1988;240(4849):219–221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases