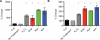Bactericidal Antibiotics Induce Toxic Metabolic Perturbations that Lead to Cellular Damage - PubMed (original) (raw)
Bactericidal Antibiotics Induce Toxic Metabolic Perturbations that Lead to Cellular Damage
Peter Belenky et al. Cell Rep. 2015.
Abstract
Understanding how antibiotics impact bacterial metabolism may provide insight into their mechanisms of action and could lead to enhanced therapeutic methodologies. Here, we profiled the metabolome of Escherichia coli after treatment with three different classes of bactericidal antibiotics (?-lactams, aminoglycosides, quinolones). These treatments induced a similar set of metabolic changes after 30 min that then diverged into more distinct profiles at later time points. The most striking changes corresponded to elevated concentrations of central carbon metabolites, active breakdown of the nucleotide pool, reduced lipid levels, and evidence of an elevated redox state. We examined potential end-target consequences of these metabolic perturbations and found that antibiotic-treated cells exhibited cytotoxic changes indicative of oxidative stress, including higher levels of protein carbonylation, malondialdehyde adducts, nucleotide oxidation, and double-strand DNA breaks. This work shows that bactericidal antibiotics induce a complex set of metabolic changes that are correlated with the buildup of toxic metabolic by-products.
Keywords: E. coli, metabolomics; antibiotics; double-strand breaks; lipid peroxidation; oxidative stress; protein carbonylation DNA damage; reactive oxygen species.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
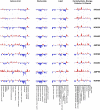
Figure 1. Bactericidal antibiotics induce broad metabolic perturbations in bacteria
Bar plots depicting fold change in the relative concentration of individual metabolites (mean value, n=5), with respect to t=0 (the UNT0 control), for E. coli treated with Amp (3 μg/mL), Kan (7.5 μg/mL), or Nor (150 ng/mL) 30, 60, and 90 minutes post antibiotic treatment. Blue indicates decreased concentration and red indicates increased concentration. Fold change values were log-transformed and plotted on a y-axis of −5 to 5.
Figure 2. Bactericidal antibiotics induce a common set of initial metabolic alterations
Hierarchical clustering of the metabolic profiling data at 30, 60, and 90 minutes after treatment with Amp (3 μg/mL), Kan, (7.5 μg/mL) or Nor (150 ng/mL). UNT0 (black bars), Kan (green bars), Amp (red bars), and Nor (blue bars). Relative concentration measurements were scaled along each row (i.e., for each metabolite) using a z-score prior to clustering, and red and blue indicate increased and decreased concentrations, respectively
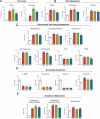
Figure 3. Bactericidal antibiotics induce a common set of metabolic perturbations
E. coli were treated with Amp (3 μg/mL), Kan (7.5 μg/mL), or Nor (150 ng/mL) for 30 minutes before samples were taken for metabolic profiling. Shown are fold changes in relative metabolic concentrations in comparison to UNT0. Data shown reflect mean ± SEM of n = 5. Statistical significance is shown (*: p ≤ 0.05; **: p ≤ 0.01; ***: p ≤ 0.001) using the untreated control for determination. A complete data set for all metabolites presented as box plots can be found in Supplemental Data S1.
Figure 4. Bactericidal antibiotics induce protein carbonylation and modification of protein by MDA
(A, B) ELISA-based determination of protein carbonylation and levels of MDA modified protein. E. coli were treated with 10 mM H2O2, 10 μg/mL Amp, 10 μg/mL Kan or 250 ng/mL Nor for 60 minutes. Statistical significance is shown (*: p ≤ 0.05) using the untreated control at 60 minutes for determination.
Figure 5. Bactericidal antibiotics induce DNA and RNA oxidation
(A, B), ELISA-based determination of 8-oxo-dG and 8-oxo-G on total DNA and RNA, respectively. E. coli were treated with 10 mM H2O2, 10 μg/mL Amp, 10 μg/mL Kan, or 250 ng/mL Nor for 60 minutes. Statistical significance is shown (*: p ≤ 0.05) using the untreated control at 60 minutes for determination.
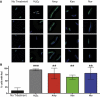
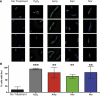
Figure 6. Bactericidal antibiotics induce double-strand breaks in E. coli
(A) Selected images showing Gam-GFP distribution (green) in untreated cells or cells treated for 2 hours with 10 mM H2O2, 2.5 μg/mL Amp, 10 μg/mL Kan, or 125 ng/mL Nor. Bacterial DNA is stained with DAPI (blue). Arrows indicate gam-GFP foci, which occur at double-strand breaks. GFP foci not colocalized with DAPI were excluded from the analysis. (B) Percent of cells with GFP foci in the indicated treatment group. Bars represent the average of three independent experiments in which 50 to 150 cells were quantitated for each condition. Error bars represent SEM. Statistical significance using Sidak's multiple comparisons test: *, p>0.05; **, p<0.01; ***, p<0.001.
Comment in
- Bactericidal antibiotics induce programmed metabolic toxicity.
Rowan AD, Cabral DJ, Belenky P. Rowan AD, et al. Microb Cell. 2016 Mar 9;3(4):178-180. doi: 10.15698/mic2016.04.493. Microb Cell. 2016. PMID: 28357350 Free PMC article.
Similar articles
- Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics.
Foti JJ, Devadoss B, Winkler JA, Collins JJ, Walker GC. Foti JJ, et al. Science. 2012 Apr 20;336(6079):315-9. doi: 10.1126/science.1219192. Science. 2012. PMID: 22517853 Free PMC article. - A common mechanism of cellular death induced by bactericidal antibiotics.
Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. Kohanski MA, et al. Cell. 2007 Sep 7;130(5):797-810. doi: 10.1016/j.cell.2007.06.049. Cell. 2007. PMID: 17803904 - On the road to bacterial cell death.
Wright GD. Wright GD. Cell. 2007 Sep 7;130(5):781-3. doi: 10.1016/j.cell.2007.08.023. Cell. 2007. PMID: 17803902 - Comparison of the changes in global gene expression of Escherichia coli induced by four bactericidal agents.
Shaw KJ, Miller N, Liu X, Lerner D, Wan J, Bittner A, Morrow BJ. Shaw KJ, et al. J Mol Microbiol Biotechnol. 2003;5(2):105-22. doi: 10.1159/000069981. J Mol Microbiol Biotechnol. 2003. PMID: 12736533 - Gut decontamination with norfloxacin and ampicillin enhances insulin sensitivity in mice.
Chou CJ, Membrez M, Blancher F. Chou CJ, et al. Nestle Nutr Workshop Ser Pediatr Program. 2008;62:127-37; discussion 137-40. doi: 10.1159/000146256. Nestle Nutr Workshop Ser Pediatr Program. 2008. PMID: 18626197 Review.
Cited by
- Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications.
Godoy-Gallardo M, Eckhard U, Delgado LM, de Roo Puente YJD, Hoyos-Nogués M, Gil FJ, Perez RA. Godoy-Gallardo M, et al. Bioact Mater. 2021 May 8;6(12):4470-4490. doi: 10.1016/j.bioactmat.2021.04.033. eCollection 2021 Dec. Bioact Mater. 2021. PMID: 34027235 Free PMC article. Review. - Protein Lipoxidation: Basic Concepts and Emerging Roles.
Viedma-Poyatos Á, González-Jiménez P, Langlois O, Company-Marín I, Spickett CM, Pérez-Sala D. Viedma-Poyatos Á, et al. Antioxidants (Basel). 2021 Feb 16;10(2):295. doi: 10.3390/antiox10020295. Antioxidants (Basel). 2021. PMID: 33669164 Free PMC article. Review. - Translation error clusters induced by aminoglycoside antibiotics.
Wohlgemuth I, Garofalo R, Samatova E, Günenç AN, Lenz C, Urlaub H, Rodnina MV. Wohlgemuth I, et al. Nat Commun. 2021 Mar 23;12(1):1830. doi: 10.1038/s41467-021-21942-6. Nat Commun. 2021. PMID: 33758186 Free PMC article. - Synergistic Lethality of a Binary Inhibitor of Mycobacterium tuberculosis KasA.
Kumar P, Capodagli GC, Awasthi D, Shrestha R, Maharaja K, Sukheja P, Li SG, Inoyama D, Zimmerman M, Ho Liang HP, Sarathy J, Mina M, Rasic G, Russo R, Perryman AL, Richmann T, Gupta A, Singleton E, Verma S, Husain S, Soteropoulos P, Wang Z, Morris R, Porter G, Agnihotri G, Salgame P, Ekins S, Rhee KY, Connell N, Dartois V, Neiditch MB, Freundlich JS, Alland D. Kumar P, et al. mBio. 2018 Dec 18;9(6):e02101-17. doi: 10.1128/mBio.02101-17. mBio. 2018. PMID: 30563908 Free PMC article.
References
- Belenky P, Collins JJ. Antioxidant Strategies to Tolerate Antibiotics. Science. 2011;334:915–916. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical