Adipokines, diabetes and atherosclerosis: an inflammatory association - PubMed (original) (raw)
Review
Adipokines, diabetes and atherosclerosis: an inflammatory association
Leandro C Freitas Lima et al. Front Physiol. 2015.
Abstract
Cardiovascular diseases can be considered the most important cause of death in diabetic population and diabetes can in turn increase the risk of cardiovascular events. Inflammation process is currently recognized as responsible for the development and maintenance of diverse chronic diseases, including diabetes and atherosclerosis. Considering that adipose tissue is an important source of adipokines, which may present anti and proinflammatory effects, the aim of this review is to explore the role of the main adipokines in the pathophysiology of diabetes and atherosclerosis, highlighting the therapeutic options that could arise from the manipulation of these signaling pathways both in humans and in translational models.
Keywords: IL-6; MCP-1; TNFα; adipokines; adiponectin; atherosclerosis; diabetes; leptin.
Figures
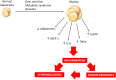
Figure 1
Over nutrition, metabolic syndrome and/or genetic predisposition may contribute to obesity development and modulate adipokine profile resulting in a low-grade inflammatory state which is associated with increased risk of insulin resistance and atherosclerosis.
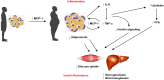
Figure 2
Increase in adipose tissue mass is related to increase in MCP-1, which favors macrophages migration to adipose tissue and the production of other adipokines such as IL-6 and TNFα. On the other hand, obese adipose tissue produces less adiponectin. Taken together, these features reduce glucose uptake in muscle cells and favors glycogenolisis and gluconeogenesis in liver, which contribute to insulin resistance and diabetes.
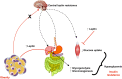
Figure 3
In obesity, despite increased levels of leptin, central actions of this adipokine that control appetite, body weight, and energy expenditure are impaired (a phenomena known as leptin resistance, represented in the figure by the crossed red arrow from adipose tissue to the brain and black arrows). Otherwise, peripheral actions of leptin to reduce insulin secretion in pancreas and its signaling pathways is muscle (represented in the figure by plain arrows from adipose tissue to pancreas and muscle) culminates in insulin resistance, hyperglycemia and diabetes.
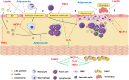
Figure 4
The process of atherogenesis can be influenced by diverse adipokines.1: Endothelial dysfunction (ED) and transmigration of LDL particles to subendothelial space can be worsen by leptin and TNFα. Adiponectin, which is reduced in obesity, recovers endothelial function. 2: Once in subendothelial space LDL is oxidized (oxLDL), which is positively related to MCP-1 levels. Leptin, IL-6, MCP-1 and TNFα increase the expression of adhesion molecules in endothelium and increase leucocyte transmigration. 3: Monocytes turn into macrophages under the stimulus of MCP-1 and phagocytes oxLDL, turning into foam cells. Adiponectin inhibits phagocytosis of oxLDL and foam cell formation. 4: IL-6 can be produced by local smooth muscle cells (SMC) under the stimulus of angiotensin II. Along with MCP-1, it increases recruitment and proliferation of SMC and extracellular matrix deposition to form a fibrous cap around a lipid-rich necrotic core. 5: Due to stimulation of matrix metalloproteinases and prothrombotic molecules, MCP-1 and leptin favors plaque rupture and thrombus formation, while adiponectin inhibits thrombosis. Periadventitial adipose tissue (PAAT) and leptin induce the production of proinflammatory adipokines. Red arrows represent proinflammatory pathways which are stimulated during obesity and contribute to atherogenesis. Blue arrows represent anti-inflammatory pathways which are inhibited during obesity since adiponectin levels are low. Green arrows represent production or stimulation of adipokine secretion.
Similar articles
- Anti-inflammatory effects of simvastatin on adipokines in type 2 diabetic patients with carotid atherosclerosis.
Hu Y, Tong G, Xu W, Pan J, Ryan K, Yang R, Shuldiner AR, Gong DW, Zhu D. Hu Y, et al. Diab Vasc Dis Res. 2009 Oct;6(4):262-8. doi: 10.1177/1479164109339966. Diab Vasc Dis Res. 2009. PMID: 20368220 Clinical Trial. - Adipokines, adiposity, and atherosclerosis.
Liu L, Shi Z, Ji X, Zhang W, Luan J, Zahr T, Qiang L. Liu L, et al. Cell Mol Life Sci. 2022 May 3;79(5):272. doi: 10.1007/s00018-022-04286-2. Cell Mol Life Sci. 2022. PMID: 35503385 Free PMC article. Review. - Translating the biology of adipokines in atherosclerosis and cardiovascular diseases: Gaps and open questions.
Ruscica M, Baragetti A, Catapano AL, Norata GD. Ruscica M, et al. Nutr Metab Cardiovasc Dis. 2017 May;27(5):379-395. doi: 10.1016/j.numecd.2016.12.005. Epub 2016 Dec 23. Nutr Metab Cardiovasc Dis. 2017. PMID: 28237179 Review. - Adipokine expression and secretion by canine adipocytes: stimulation of inflammatory adipokine production by LPS and TNFalpha.
Ryan VH, German AJ, Wood IS, Hunter L, Morris P, Trayhurn P. Ryan VH, et al. Pflugers Arch. 2010 Aug;460(3):603-16. doi: 10.1007/s00424-010-0845-x. Epub 2010 May 15. Pflugers Arch. 2010. PMID: 20473515 - Adipose tissue and adipokines: for better or worse.
Guerre-Millo M. Guerre-Millo M. Diabetes Metab. 2004 Feb;30(1):13-9. doi: 10.1016/s1262-3636(07)70084-8. Diabetes Metab. 2004. PMID: 15029093 Review.
Cited by
- Physical exercise, obesity, inflammation and neutrophil extracellular traps (NETs): a review with bioinformatics analysis.
Valeria Oliveira de Sousa B, de Freitas DF, Monteiro-Junior RS, Mendes IHR, Sousa JN, Guimarães VHD, Santos SHS. Valeria Oliveira de Sousa B, et al. Mol Biol Rep. 2021 May;48(5):4625-4635. doi: 10.1007/s11033-021-06400-2. Epub 2021 May 20. Mol Biol Rep. 2021. PMID: 34014471 Review. - Ablation of Galectin-12 Inhibits Atherosclerosis through Enhancement of M2 Macrophage Polarization.
Lin ES, Hsu YA, Chang CY, Lin HJ, Chen CS, Wan L. Lin ES, et al. Int J Mol Sci. 2020 Jul 31;21(15):5511. doi: 10.3390/ijms21155511. Int J Mol Sci. 2020. PMID: 32752134 Free PMC article. - Serum levels of CTRP3 in diabetic nephropathy and its relationship with insulin resistance and kidney function.
Moradi N, Fadaei R, Khamseh ME, Nobakht A, Rezaei MJ, Aliakbary F, Vatannejad A, Hosseini J. Moradi N, et al. PLoS One. 2019 Apr 22;14(4):e0215617. doi: 10.1371/journal.pone.0215617. eCollection 2019. PLoS One. 2019. PMID: 31009504 Free PMC article. - A Disintegrin and Metalloprotease 17 in the Cardiovascular and Central Nervous Systems.
Xu J, Mukerjee S, Silva-Alves CR, Carvalho-Galvão A, Cruz JC, Balarini CM, Braga VA, Lazartigues E, França-Silva MS. Xu J, et al. Front Physiol. 2016 Oct 18;7:469. doi: 10.3389/fphys.2016.00469. eCollection 2016. Front Physiol. 2016. PMID: 27803674 Free PMC article. Review. - Cancer and comorbidity: The role of leptin in breast cancer and associated pathologies.
Ray A. Ray A. World J Clin Cases. 2018 Oct 26;6(12):483-492. doi: 10.12998/wjcc.v6.i12.483. World J Clin Cases. 2018. PMID: 30397604 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous