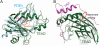Targeting the Central Pocket in Human Transcription Factor TEAD as a Potential Cancer Therapeutic Strategy - PubMed (original) (raw)
Targeting the Central Pocket in Human Transcription Factor TEAD as a Potential Cancer Therapeutic Strategy
Ajaybabu V Pobbati et al. Structure. 2015.
Abstract
The human TEAD family of transcription factors (TEAD1-4) is required for YAP-mediated transcription in the Hippo pathway. Hyperactivation of TEAD's co-activator YAP contributes to tissue overgrowth and human cancers, suggesting that pharmacological interference of TEAD-YAP activity may be an effective strategy for anticancer therapy. Here we report the discovery of a central pocket in the YAP-binding domain (YBD) of TEAD that is targetable by small-molecule inhibitors. Our X-ray crystallography studies reveal that flufenamic acid, a non-steroidal anti-inflammatory drug (NSAID), binds to the central pocket of TEAD2 YBD. Our biochemical and functional analyses further demonstrate that binding of NSAIDs to TEAD inhibits TEAD-YAP-dependent transcription, cell migration, and proliferation, indicating that the central pocket is important for TEAD function. Therefore, our studies discover a novel way of targeting TEAD transcription factors and set the stage for therapeutic development of specific TEAD-YAP inhibitors against human cancers.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
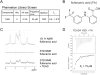
Figure 1. The YAP-binding Domain of TEADs Has a Central Pocket
(A) Domain architecture of TEAD1 and YAP. All the four TEAD genes have a N-terminal DNA-binding TEA domain and a C-terminal YAP-binding domain (YBD). YAP has an N-terminal TEAD-binding domain (TBD) followed by one or two WW domains and a C-terminal activation domain. (B) The crystal structure of the TEAD-YAP complex (PDB code 3KYS) reveals three interfaces between TEAD and YAP. TEAD YBD is colored green and YAP TBD is colored magenta. TEAD YBD has a large pocket in the center (red circle); the hydrophobic volume of this pocket is shown in yellow. On both ends of this pocket are hydrophilic areas, shown in red and blue that could be used to improve the specificity of TEAD-binding drugs. All structural figures were generated with Schrödinger software suite or Chimera (UCSF). (C) Isothermal titration calorimetry (ITC) showing heat response during TEAD-YAP interaction. The binding affinity (Kd) between the YAP peptide 1 and TEAD was indicated. (D) Sequences and binding affinities of the YAP peptides used in this study. Residues from Interface 2 and 3 are underlined. (E) Summary of the results from fragment library screen.
Figure 2. Identification of Flufenamic Acid as a TEAD-binding Drug
(A) Summary of the results from Pharmakon library screen. (B) Structure of flufenamic acid (FA). (C) Saturation-Transfer Difference (STD) NMR spectra of FA showing a conspicuous change after the addition of TEAD. Upper panel shows the 1D 1H NMR spectrum of free FA. Middle and lower panels are STD spectra of FA in the absence and presence of TEAD. (D) ITC measurement of the affinity (Kd) between TEAD and FA.
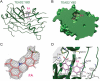
Figure 3. Structure of the TEAD2-flufenamic acid Complex
(A) Crystal structure of the TEAD2-FA complex reveals that FA binds to the central pocket in TEAD. TEAD2 YBD is colored green. FA is shown as pink sticks. (B) A cross-section of the surface drawing of TEAD2 YBD shows FA occupying the central pocket. (C) Simulated annealing omit map contoured at 1 σ for FA. (D) Detailed view of the TEAD-FA interaction. FA and FA-contacting residues in the central pocket are shown as pink and grey sticks, respectively. The hydrogen bond between FA and C380 is shown as a green dash line.
Figure 4. Specificity of the TEAD-fenamate Interactions
(A) Crystal structure of the TEAD2-BFA complex. Molecule 1 of BFA binds to the central pocket. Molecule 2 of BFA binds to the YAP-binding Interface 3 region of TEAD. TEAD YBD is colored green. BFA are shown as pink sticks. (B) Monitoring TEAD-YAP interaction using TEAD4 YBD and a fluorescent-labeled YAP peptide. The native gel shows that TEAD wild-type (WT) and TEAD double mutant (dm) interact with YAP with similar efficiency. (C) The affinity (Kd) between flufenamic acid and TEAD dm was measured by ITC. (D) Structures of FA analogs and their binding affinities towards TEAD. (E) ITC measurement of the affinity (Kd) between TEAD and niflumic acid (NA). (F) The minimal energy conformations of FA and NA are shown as grey sticks. The conformation of FA in the crystal structure of TEAD2-FA and the docked conformation of NA are shown as pink sticks.
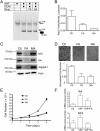
Figure 5. Biological Consequence of Fenamate Binding to TEADs
(A) Native gel showing that the binding of TEAD to fluorescently labeled YAP peptide is unaffected in the presence of the flufenamate drugs. (B) Significant reduction in the TEAD reporter activity was observed after the treatment of cells with flufenamic acid (FA) and niflumic acid (NA). (C) The expression of Hippo-responsive genes, such as NF2, Axl and Jagged-1, were greatly reduced after flufenamic acid (FA) treatment. However, no significant decrease was observed when the cells were treated with mefanamic acid (MA). (D) Migration of HEK293 cells was measured after FA and NA treatments using a transwell assay. The cells that migrate across the membrane were visualized using crystal violet staining. The quantification is shown below. (E) Proliferation of HEK293 cells was measured after treatment with FA and NA. (F) The qPCR data shows that flufenamic acid (FA) reduces the expression of genes, such as Axl and NF2 that are stimulated upon YAP overexpression. All the error bars in the figure represent SD.
Figure 6. TEAD and PDEδ Have a Similar Hydrophobic Central Pocket
(A) Superposition of TEAD-FA and PDEδ-farnesyl (PDB code 3T5I) complexes. Farnesyl (shown as orange sticks) and FA (shown as pink sticks) occupy the central pocket of PDEδ and TEAD, respectively. (B) A structural model to show that a fenamate analog (grey sticks) extends from the central pocket to interfere with TEAD-YAP interaction (red circle). A propyne substituent was added ortho to the acid of FA and the compound was docked onto the TEAD1-YAP complex (PDB code 3KYS). Bulky substituents in this position may disrupt YAP binding.
Similar articles
- Structural and functional analysis of the YAP-binding domain of human TEAD2.
Tian W, Yu J, Tomchick DR, Pan D, Luo X. Tian W, et al. Proc Natl Acad Sci U S A. 2010 Apr 20;107(16):7293-8. doi: 10.1073/pnas.1000293107. Epub 2010 Apr 5. Proc Natl Acad Sci U S A. 2010. PMID: 20368466 Free PMC article. - Discovery of a new class of reversible TEA domain transcription factor inhibitors with a novel binding mode.
Hu L, Sun Y, Liu S, Erb H, Singh A, Mao J, Luo X, Wu X. Hu L, et al. Elife. 2022 Nov 18;11:e80210. doi: 10.7554/eLife.80210. Elife. 2022. PMID: 36398861 Free PMC article. - Structural and ligand-binding analysis of the YAP-binding domain of transcription factor TEAD4.
Li Y, Liu S, Ng EY, Li R, Poulsen A, Hill J, Pobbati AV, Hung AW, Hong W, Keller TH, Kang C. Li Y, et al. Biochem J. 2018 Jun 26;475(12):2043-2055. doi: 10.1042/BCJ20180225. Biochem J. 2018. PMID: 29760238 - Direct Inhibition of the YAP : TEAD Interaction: An Unprecedented Drug Discovery Challenge.
Chène P. Chène P. ChemMedChem. 2024 Oct 1;19(19):e202400361. doi: 10.1002/cmdc.202400361. Epub 2024 Jul 24. ChemMedChem. 2024. PMID: 38863297 Review. - An evolutionary, structural and functional overview of the mammalian TEAD1 and TEAD2 transcription factors.
Landin-Malt A, Benhaddou A, Zider A, Flagiello D. Landin-Malt A, et al. Gene. 2016 Oct 10;591(1):292-303. doi: 10.1016/j.gene.2016.07.028. Epub 2016 Jul 14. Gene. 2016. PMID: 27421669 Free PMC article. Review.
Cited by
- Mechanoregulation and pathology of YAP/TAZ via Hippo and non-Hippo mechanisms.
Dobrokhotov O, Samsonov M, Sokabe M, Hirata H. Dobrokhotov O, et al. Clin Transl Med. 2018 Aug 13;7(1):23. doi: 10.1186/s40169-018-0202-9. Clin Transl Med. 2018. PMID: 30101371 Free PMC article. Review. - YAP signaling is involved in WDR1-regulated proliferation and migration of non-small-cell lung cancer cells.
An R, Wang J, Chen X, Xu R, Hu J, Liu Z, Wei C, Zhang C, Yuan B. An R, et al. Exp Biol Med (Maywood). 2022 Sep;247(18):1619-1629. doi: 10.1177/15353702221110645. Epub 2022 Jul 21. Exp Biol Med (Maywood). 2022. PMID: 35861209 Free PMC article. - Toward the Discovery of a Novel Class of YAP⁻TEAD Interaction Inhibitors by Virtual Screening Approach Targeting YAP⁻TEAD Protein⁻Protein Interface.
Gibault F, Coevoet M, Sturbaut M, Farce A, Renault N, Allemand F, Guichou JF, Drucbert AS, Foulon C, Magnez R, Thuru X, Corvaisier M, Huet G, Chavatte P, Melnyk P, Bailly F, Cotelle P. Gibault F, et al. Cancers (Basel). 2018 May 8;10(5):140. doi: 10.3390/cancers10050140. Cancers (Basel). 2018. PMID: 29738494 Free PMC article. - The HIPPO pathway in gynecological malignancies.
Wang D, He J, Dong J, Meyer TF, Xu T. Wang D, et al. Am J Cancer Res. 2020 Feb 1;10(2):610-629. eCollection 2020. Am J Cancer Res. 2020. PMID: 32195031 Free PMC article. Review. - TEAD transcription factor family emerges as a promising therapeutic target for oral squamous cell carcinoma.
Wang S, Shao D, Gao X, Zhao P, Kong F, Deng J, Yang L, Shang W, Sun Y, Fu Z. Wang S, et al. Front Immunol. 2024 Oct 4;15:1480701. doi: 10.3389/fimmu.2024.1480701. eCollection 2024. Front Immunol. 2024. PMID: 39430767 Free PMC article. Review.
References
- Bao Y, Nakagawa K, Yang Z, Ikeda M, Withanage K, Ishigami-Yuasa M, Okuno Y, Hata S, Nishina H, Hata Y. A cell-based assay to screen stimulators of the Hippo pathway reveals the inhibitory effect of dobutamine on the YAP-dependent gene transcription. J Biochem. 2011;150:199–208. - PubMed
- Begley DW, Moen SO, Pierce PG, Zartler ER. Saturation transfer difference NMR for fragment screening. Curr Protoc Chem Biol. 2013;5:251–268. - PubMed
- Chan SW, Lim CJ, Guo K, Ng CP, Lee I, Hunziker W, Zeng Q, Hong W. A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Res. 2008;68:2592–2598. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases