Nectar uptake in bats using a pumping-tongue mechanism - PubMed (original) (raw)
Nectar uptake in bats using a pumping-tongue mechanism
Marco Tschapka et al. Sci Adv. 2015.
Abstract
Many insects use nectar as their principal diet and have mouthparts specialized in nectarivory, whereas most nectar-feeding vertebrates are opportunistic users of floral resources and only a few species show distinct morphological specializations. Specialized nectar-feeding bats extract nectar from flowers using elongated tongues that correspond to two vastly different morphologies: Most species have tongues with hair-like papillae, whereas one group has almost hairless tongues that show distinct lateral grooves. Recent molecular data indicate a convergent evolution of groove- and hair-tongued bat clades into the nectar-feeding niche. Using high-speed video recordings on experimental feeders, we show distinctly divergent nectar-feeding behavior in clades. Grooved tongues are held in contact with nectar for the entire duration of visit as nectar is pumped into the mouths of hovering bats, whereas hairy tongues are used in conventional sinusoidal lapping movements. Bats with grooved tongues use a specific fluid uptake mechanism not known from any other mammal. Nectar rises in semiopen lateral grooves, probably driven by a combination of tongue deformation and capillary action. Extraction efficiency declined for both tongue types with a similar slope toward deeper nectar levels. Our results highlight a novel drinking mechanism in mammals and raise further questions on fluid mechanics and ecological niche partitioning.
Keywords: Chiroptera; Glossophaginae; Lonchophyllinae; adaptations; animal-plant interaction; nectar feeding; pollination.
Figures
Fig. 1. Tongue movement.
(Left) Extended tongues of drinking: (top) G. soricina (Glossophaginae) and (bottom) L. robusta (Lonchophyllinae). Although the tongue of G. soricina is covered by long filiform papillae, the tongue of L. robusta shows a distinct lateral canal. (Right) Movement patterns of the tongue tips of G. soricina (A) and L. robusta (B) drinking at a feeder offering honey water at 20 mm below the opening. The tongue of L. robusta submerges in the fluid at the beginning of the visit and stays there with only small movements, whereas the tongue of G. soricina extends and retracts repeatedly in stereotypic lapping movements. Interruptions of Glossophaga curves near the upper rim of the feeder represent the total retraction of the tongue into the mouth. (Left, center) Phylogenetic relations between the two groups [modified from (14, 27)] (figs. S1 and S2 and videos S1 to S4).
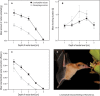
Fig. 2. Feeding behavior.
(A) The amount of nectar extracted after each visit decreases steadily toward deeper levels. (B) Hovering duration in G. soricina and L. robusta increases when bats have to reach deeper into the feeder. The final decline occurs when bats abort their visit upon reaching the limit of their tongue extension capability. (C) Standardized extraction efficiency in both species decreases at a very similar slope. All figures are presented as mean ±1 SE. (D) L. robusta visiting a bromeliad flower (Werauhia sp.). Photo was taken at the Bocas del Toro Field Station of the Smithsonian Tropical Research Institute on March 2009.
Similar articles
- Morphological specialization influences nectar extraction efficiency of sympatric nectar-feeding bats.
Gonzalez-Terrazas TP, Medellin RA, Knörnschild M, Tschapka M. Gonzalez-Terrazas TP, et al. J Exp Biol. 2012 Nov 15;215(Pt 22):3989-96. doi: 10.1242/jeb.068494. Epub 2012 Aug 16. J Exp Biol. 2012. PMID: 22899529 - Morphological specialization to nectarivory in Phyllostomus discolor (Wagner, 1843) (Chiroptera: Phyllostomidae).
Quinche LL, Santana SE, Rico-Guevara A. Quinche LL, et al. Anat Rec (Hoboken). 2023 Nov;306(11):2830-2841. doi: 10.1002/ar.25147. Epub 2022 Dec 27. Anat Rec (Hoboken). 2023. PMID: 36573585 - Feeding adaptations in the hairs and tongues of nectar-feeding bats.
Howell DJ, Hodgkin N. Howell DJ, et al. J Morphol. 1976 Mar;148(3):329-39. doi: 10.1002/jmor.1051480305. J Morphol. 1976. PMID: 1255733 - Nectar Feeding by a Honey Bee's Hairy Tongue: Morphology, Dynamics, and Energy-Saving Strategies.
Wang H, Wu Z, Zhao J, Wu J. Wang H, et al. Insects. 2021 Aug 24;12(9):762. doi: 10.3390/insects12090762. Insects. 2021. PMID: 34564203 Free PMC article. Review. - Coloured nectar: distribution, ecology, and evolution of an enigmatic floral trait.
Hansen DM, Olesen JM, Mione T, Johnson SD, Müller CB. Hansen DM, et al. Biol Rev Camb Philos Soc. 2007 Feb;82(1):83-111. doi: 10.1111/j.1469-185X.2006.00005.x. Biol Rev Camb Philos Soc. 2007. PMID: 17313525 Review.
Cited by
- Feeding efficiency of two coexisting nectarivorous bat species (Phyllostomidae: Glossophaginae) at flowers of two key-resource plants.
Bechler JP, Steiner K, Tschapka M. Bechler JP, et al. PLoS One. 2024 Jun 26;19(6):e0303227. doi: 10.1371/journal.pone.0303227. eCollection 2024. PLoS One. 2024. PMID: 38924018 Free PMC article. - Bromeliads going batty: pollinator partitioning among sympatric chiropterophilous Bromeliaceae.
Aguilar-Rodríguez PA, Tschapka M, García-Franco JG, Krömer T, MacSwiney G MC. Aguilar-Rodríguez PA, et al. AoB Plants. 2019 Mar 12;11(2):plz014. doi: 10.1093/aobpla/plz014. eCollection 2019 Apr. AoB Plants. 2019. PMID: 31186827 Free PMC article. - The Tongue in Three Species of Lemurs: Flower and Nectar Feeding Adaptations.
Pastor JF, Muchlinski MN, Potau JM, Casado A, García-Mesa Y, Vega JA, Cabo R. Pastor JF, et al. Animals (Basel). 2021 Sep 27;11(10):2811. doi: 10.3390/ani11102811. Animals (Basel). 2021. PMID: 34679832 Free PMC article. - Honey bees switch mechanisms to drink deep nectar efficiently.
Wei J, Rico-Guevara A, Nicolson SW, Brau F, Damman P, Gorb SN, Wu Z, Wu J. Wei J, et al. Proc Natl Acad Sci U S A. 2023 Jul 25;120(30):e2305436120. doi: 10.1073/pnas.2305436120. Epub 2023 Jul 17. Proc Natl Acad Sci U S A. 2023. PMID: 37459520 Free PMC article. - Sucking or lapping: facultative feeding mechanisms in honeybees (Apis mellifera).
Wei J, Huo Z, Gorb SN, Rico-Guevara A, Wu Z, Wu J. Wei J, et al. Biol Lett. 2020 Aug;16(8):20200449. doi: 10.1098/rsbl.2020.0449. Epub 2020 Aug 12. Biol Lett. 2020. PMID: 32780979 Free PMC article.
References
- Baker H. G., Baker I., Hodges S. A., Sugar composition of nectars and fruits consumed by birds and bats in the tropics and subtropics. Biotropica 30, 559–586 (1998).
- S. W. Nicolson, R. W. Thornburg, in Nectaries and Nectar, S. W. Nicolson, M. Nepi, E. Pacini, Eds. (Springer, Dordrecht, Netherlands, 2007), pp. 215–264.
- Olesen J. M., Valido A., Lizards as pollinators and seed dispersers: An island phenomenon. Trends Ecol. Evol. 18, 177–181 (2003).
- T. H. Fleming, W. J. Kress, The Ornaments of Life: Coevolution and Conservation in the Tropics (University of Chicago Press, Chicago, IL, 2013).
- Bradshaw D., Bradshaw F., The physiology of the honey possum, Tarsipes rostratus, a small marsupial with a suite of highly specialised characters: A review. J. Comp. Physiol. B 182, 469–489 (2012). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources