SGLT2 inhibitors - an insulin-independent therapeutic approach for treatment of type 2 diabetes: focus on canagliflozin - PubMed (original) (raw)
Review
SGLT2 inhibitors - an insulin-independent therapeutic approach for treatment of type 2 diabetes: focus on canagliflozin
Jochen Seufert. Diabetes Metab Syndr Obes. 2015.
Abstract
Despite the availability of a great variety of medications, a significant proportion of people with type 2 diabetes mellitus (T2DM) are not able to achieve or maintain adequate glycemic control. Beyond improved glucose control, novel treatments would ideally provide a reduction of cardiovascular risk, with a favorable impact on excess weight, and a low intrinsic hypoglycemia risk, as well as a synergistic mechanism of action for broad combination therapy. With the development of sodium glucose cotransporter 2 (SGLT2) inhibitors, an antidiabetic pharmacologic option has recently become available that comes close to meeting these requirements. For the first time, SGLT2 inhibitors offer a therapeutic approach acting directly on the kidneys without requiring insulin secretion or action. Canagliflozin, dapagliflozin, and empagliflozin are the SGLT2 inhibitors approved to date. Taken once a day, these medications can be combined with all other antidiabetic medications including insulin, due to their insulin-independent mechanism of action, with only a minimal risk of hypoglycemia. SGLT2 inhibitors provide additional reductions in body weight and blood pressure due to the therapeutically induced excretion of glucose and sodium through the kidneys. These "concomitant effects" are particularly interesting with regard to the increased cardiovascular risk in T2DM. In many cases, T2DM treatment requires a multidimensional approach where the treatment goals have to be adapted to the individual patient. While there is a consensus on the use of metformin as a first-line drug therapy, various antidiabetics are used for treatment intensification. New mechanisms of action like that of SGLT2 inhibitors such as canagliflozin, which can be used both in early and late stages of diabetes, are a welcome addition to expand the treatment options for patients at every stage of T2DM. The efficacy and tolerability of canagliflozin have been tested in an extensive clinical trial program described in this review article.
Keywords: canagliflozin; dapagliflozin; empagliflozin; sodium glucose cotransporter 2 (SGLT2) inhibitor; type 2 diabetes.
Figures
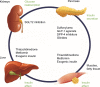
Figure 1
Pharmacologic options for the treatment of T2DM: SGLT2 inhibition in the kidneys complements existing treatment options through an insulin-independent mechanism of action. Notes: Adapted from Seufert J. SGLT-2 inhibition with canagliflozin: a new option for the treatment of type 2 diabetes. Dtsch Med Wochenschr. 2014;139(Suppl 2): S52–S58. © Georg Thieme Verlag KG. Abbreviations: T2DM, type 2 diabetes mellitus; SGLT2, sodium glucose cotransporter 2; GLP-1, glucagon-like peptide-1; DPP-4, dipeptidyl peptidase-4.
Figure 2
Phase III trial program with canagliflozin. Notes: Data from published studies.,,–, Abbreviations: OAD, oral antidiabetic; MET, metformin; PBO, placebo; SITA, sitagliptin; PIO, pioglitazone; CANVAS, CANagliflozin cardioVascular Assessment Study; SU, sulfonylurea; GLIM, glimepiride; T2DM, type 2 diabetes mellitus; CV, cardiovascular; eGFR, estimated glomerular filtration rate.
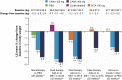
Figure 3
Percent change in body weight as a concomitant effect of SGLT2 inhibition with canagliflozin 100 mg and 300 mg in a direct comparison with placebo, glimepiride, and sitagliptin: examples from clinical Phase III trials on canagliflozin monotherapy, dual combination therapy, triple combination therapy, and in combination with insulin. Notes: *P<0.001 vs PBO. **P<0.0001 vs GLIM. ***P<0.001 vs SITA. Abbreviations: SGLT2, sodium glucose cotransporter 2; CANA, canagliflozin; PBO, placebo; GLIM, glimepiride; SITA, sitagliptin; LS, least squares; MET, metformin; SU, sulfonylurea; OAD, oral antidiabetic.
Figure 4
Changes to systolic blood pressure levels as a concomitant effect of SGLT2 inhibition with canagliflozin 100 mg and 300 mg in a direct comparison with placebo, glimepiride, and sitagliptin: examples from clinical Phase III trials with canagliflozin monotherapy, dual combination therapy, triple combination therapy, and in combination with insulin. Notes: *P<0.001 vs PBO. **P<0.001 vs SITA. Abbreviations: SGLT2, sodium glucose cotransporter 2; CANA, canagliflozin; PBO, placebo; GLIM, glimepiride; SITA, sitagliptin; LS, least squares; BP, blood pressure; MET, metformin; SU, sulfonylurea; OAD, oral antidiabetic.
Similar articles
- Sodium-glucose co-transporter 2 (SGLT2) inhibitors: a growing class of antidiabetic agents.
Vivian EM. Vivian EM. Drugs Context. 2014 Dec 19;3:212264. doi: 10.7573/dic.212264. eCollection 2014. Drugs Context. 2014. PMID: 25598831 Free PMC article. Review. - Ipragliflozin and other sodium-glucose cotransporter-2 (SGLT2) inhibitors in the treatment of type 2 diabetes: preclinical and clinical data.
Kurosaki E, Ogasawara H. Kurosaki E, et al. Pharmacol Ther. 2013 Jul;139(1):51-9. doi: 10.1016/j.pharmthera.2013.04.003. Epub 2013 Apr 4. Pharmacol Ther. 2013. PMID: 23563279 Review. - Pleiotropic effects of SGLT2 inhibitors beyond the effect on glycemic control.
Satoh H. Satoh H. Diabetol Int. 2018 Aug 14;9(4):212-214. doi: 10.1007/s13340-018-0367-x. eCollection 2018 Oct. Diabetol Int. 2018. PMID: 30603370 Free PMC article. - Update on SGLT2 Inhibitors-New Data Released at the American Diabetes Association.
Lee S. Lee S. Crit Pathw Cardiol. 2017 Sep;16(3):93-95. doi: 10.1097/HPC.0000000000000125. Crit Pathw Cardiol. 2017. PMID: 28742644 Review. - Sodium glucose transporter protein 2 inhibitors: focusing on the kidney to treat type 2 diabetes.
Peene B, Benhalima K. Peene B, et al. Ther Adv Endocrinol Metab. 2014 Oct;5(5):124-36. doi: 10.1177/2042018814553965. Ther Adv Endocrinol Metab. 2014. PMID: 25419452 Free PMC article. Review.
Cited by
- Effects of sodium-glucose cotransporter 2 inhibitors on cardiovascular and cerebrovascular diseases: a meta-analysis of controlled clinical trials.
Wang F, Li C, Cui L, Gu S, Zhao J, Wang H. Wang F, et al. Front Endocrinol (Lausanne). 2024 Aug 23;15:1436217. doi: 10.3389/fendo.2024.1436217. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39247919 Free PMC article. - Pleiotropic Effects of Sodium-Glucose Cotransporter-2 Inhibitors in Cardiovascular Disease and Chronic Kidney Disease.
Rastogi A, Januzzi JL Jr. Rastogi A, et al. J Clin Med. 2023 Apr 12;12(8):2824. doi: 10.3390/jcm12082824. J Clin Med. 2023. PMID: 37109162 Free PMC article. Review. - Bioactive Substances and Biological Functions in Malus hupehensis: A Review.
Li P, Tan J, Xiao M, Cai X, Xue H, Yu H. Li P, et al. Molecules. 2023 Jan 9;28(2):658. doi: 10.3390/molecules28020658. Molecules. 2023. PMID: 36677713 Free PMC article. Review. - Renal outcomes with sodium-glucose cotransporters 2 inhibitors.
Sun X, Wang G. Sun X, et al. Front Endocrinol (Lausanne). 2022 Dec 1;13:1063341. doi: 10.3389/fendo.2022.1063341. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36531469 Free PMC article. Review.
References
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–149. - PubMed
- de Pablos-Velasco P, Parhofer KG, Bradley C, et al. Current level of glycaemic control and its associated factors in patients with type 2 diabetes across Europe: data from the PANORAMA study. Clin Endocrinol (Oxf) 2014;80(1):47–56. - PubMed
- Bundesärztekammer (BÄK), Kassenärztliche Bundesvereinigung (KBV), Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF). Nationale VersorgungsLeitlinie Therapie des Typ-2-Diabetes – Langfassung, 1. Auflage. Version 3. 2013, zuletzt geändert: April 2014. [National Patient-Centered Guideline: Therapy of Type 2 Diabetes – Long Version, 1. Edition, Version 3. 2013, latest adaptation: April 2014] [Accessed November 19, 2014]. Available from: http://www.versorgungsleitlinien.de/themen/diabetes2/dm2_therapie. German. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources