Gut microbiome in health and disease: Linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases - PubMed (original) (raw)
Review
Gut microbiome in health and disease: Linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases
Shivani Ghaisas et al. Pharmacol Ther. 2016 Feb.
Abstract
The gut microbiome comprises the collective genome of the trillions of microorganisms residing in our gastrointestinal ecosystem. The interaction between the host and its gut microbiome is a complex relationship whose manipulation could prove critical to preventing or treating not only various gut disorders, like irritable bowel syndrome (IBS) and ulcerative colitis (UC), but also central nervous system (CNS) disorders, such as Alzheimer's and Parkinson's diseases. The purpose of this review is to summarize what is known about the gut microbiome, how it is connected to the development of disease and to identify the bacterial and biochemical targets that should be the focus of future research. Understanding the mechanisms behind the activity and proliferation of the gut microbiome will provide us new insights that may pave the way for novel therapeutic strategies.
Keywords: Autism; Environmental factors; Gut microbiota; Manganese; Metals; Microbiome–gut–brain axis; Neurological diseases; Neurotoxicity; Parkinson's disease.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest: A.G.K. is a shareholder of PK Biosciences Corporation (Ames, IA), which is interested in translating mechanistic studies into therapies targeting kinase signaling. The other authors have no conflicts of interest.
Figures
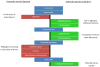
Figure 1
Schematic representation of potentially harmful and potentially beneficial bacteria present in the gut microbiome. Pro-biotic bacteria such as Lactobacillus and Bifidobacteria modulate the gut environment by releasing bioactive compounds that enhance enteric epithelial barrier function as well as by competitively binding to the epithelium thus outcompeting pathogenic bacteria. Eubacterium rectale and Fusobacterium produce fatty acids such as acetic acid, propionate and butyrate that are important as an energy source for intestinal epithelial cells as well as for modulating mucosal immune responses. In contrast, higher counts of bacteria such as Staphylococcus and Pseudomonas are seen in various metabolic disorders such as diabetes and obesity. Clostridum tetani spores are resistant to the acidic environment of the stomach and to regular antibiotic treatments. Production of the tetanus toxin by these bacteria is thought to contribute to the “leaky gut syndrome” prevalent in autistic children. E.coli is a common commensal of the gut microbiome. However, certain serotypes are pathogenic and are known to cause gastroenteritis and urinary tract infections.
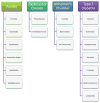
Figure 2
Bacterial species relevant to research on Autism, Parkinson’s disease, Alzheimer’s disorder, and Type-2 Diabetes. Clostridia are prevalent in many diseases and notably, presence of C. tetani in the gut microbiome of autistic children may contribute the “leaky gut” syndrome seen in these children. Lower counts of probiotic bacteria like Lactobacillus are also seen in many disorders. Understanding the differences in bacterial species seen in healthy and diseased states is crucial to understanding their importance to host health.
Figure 3
The Gut-Brain axis: Bidirectional signaling between the gastrointestinal tract (GIT) and central nervous system (CNS) occurs through spinal afferents and the vagus nerve. This mode of communication is thought to occur through peptides such as Neuropeptide Y (NpY), Cholecystokinin (CcK), ghrelin, leptin as well as by neurotransmitters like dopamine (DA), serotonin (5-HT), GABA, acetylcholine (Ach) and glutamate. Human and animal studies of various diseases demonstrate that these two systems are not exclusive of one another but do in fact show some parallels in terms of expression of pro-inflammatory cytokines and altered physiological functions.
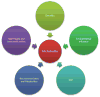
Figure 4
Factors affecting gut microbiota composition: The host-microbiota interaction is a complex and dynamic symbiosis. While diet and environment are well known factors affecting the populations of different phyla in the gut, some reports suggest that the host’s genetic composition might pre-dispose the continued growth of certain microbiota. The microbiome population can also be modulated by the presence of neurotransmitters and metabolites secreted by the host.
Similar articles
- The Gut Microbiota and Alzheimer's Disease.
Jiang C, Li G, Huang P, Liu Z, Zhao B. Jiang C, et al. J Alzheimers Dis. 2017;58(1):1-15. doi: 10.3233/JAD-161141. J Alzheimers Dis. 2017. PMID: 28372330 Review. - Can microbiota research change our understanding of neurodegenerative diseases?
Scheperjans F. Scheperjans F. Neurodegener Dis Manag. 2016 Apr;6(2):81-5. doi: 10.2217/nmt-2015-0012. Epub 2016 Apr 1. Neurodegener Dis Manag. 2016. PMID: 27033377 Review. No abstract available. - Microbiota-Brain-Gut Axis and Neurodegenerative Diseases.
Quigley EMM. Quigley EMM. Curr Neurol Neurosci Rep. 2017 Oct 17;17(12):94. doi: 10.1007/s11910-017-0802-6. Curr Neurol Neurosci Rep. 2017. PMID: 29039142 Review. - Gut microbiota in neurodegenerative disorders.
Roy Sarkar S, Banerjee S. Roy Sarkar S, et al. J Neuroimmunol. 2019 Mar 15;328:98-104. doi: 10.1016/j.jneuroim.2019.01.004. Epub 2019 Jan 9. J Neuroimmunol. 2019. PMID: 30658292 Review. - Global research trends in microbiome-gut-brain axis during 2009-2018: a bibliometric and visualized study.
Zyoud SH, Smale S, Waring WS, Sweileh WM, Al-Jabi SW. Zyoud SH, et al. BMC Gastroenterol. 2019 Aug 30;19(1):158. doi: 10.1186/s12876-019-1076-z. BMC Gastroenterol. 2019. PMID: 31470803 Free PMC article.
Cited by
- Effect of Bacteriocins on the Intestinal Microbiota.
Doğan S, Koç TY, Karadayı M. Doğan S, et al. Eurasian J Med. 2023 Dec;55(1):165-169. doi: 10.5152/eurasianjmed.2023.23393. Eurasian J Med. 2023. PMID: 39128034 Free PMC article. - Identifying Microbiota Signature and Functional Rules Associated With Bacterial Subtypes in Human Intestine.
Chen L, Li D, Shao Y, Wang H, Liu Y, Zhang Y. Chen L, et al. Front Genet. 2019 Nov 15;10:1146. doi: 10.3389/fgene.2019.01146. eCollection 2019. Front Genet. 2019. PMID: 31803234 Free PMC article. - Role of Gut Microbiota in Neurological Disorders and Its Therapeutic Significance.
Tiwari P, Dwivedi R, Bansal M, Tripathi M, Dada R. Tiwari P, et al. J Clin Med. 2023 Feb 19;12(4):1650. doi: 10.3390/jcm12041650. J Clin Med. 2023. PMID: 36836185 Free PMC article. Review. - Altered Gut Microbial Load and Immune Activation in a Drosophila Model of Human Tauopathy.
Rydbom J, Kohl H, Hyde VR, Lohr KM. Rydbom J, et al. Front Neurosci. 2021 Nov 2;15:731602. doi: 10.3389/fnins.2021.731602. eCollection 2021. Front Neurosci. 2021. PMID: 34803581 Free PMC article.
References
- Afeseh Ngwa H, Kanthasamy A, Anantharam V, Song C, Witte T, Houk R, Kanthasamy AG. Vanadium induces dopaminergic neurotoxicity via protein kinase Cdelta dependent oxidative signaling mechanisms: relevance to etiopathogenesis of Parkinson’s disease. Toxicol Appl Pharmacol. 2009;240:273–285. - PMC - PubMed
- Aoyama M, Kotani J, Usami M. Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition. 2010;26:653–661. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 ES019267/ES/NIEHS NIH HHS/United States
- ES10586/ES/NIEHS NIH HHS/United States
- R01 NS074443/NS/NINDS NIH HHS/United States
- ES19267/ES/NIEHS NIH HHS/United States
- R01 ES010586/ES/NIEHS NIH HHS/United States
- NS 074443/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical