Warning coloration can be disruptive: aposematic marginal wing patterning in the wood tiger moth - PubMed (original) (raw)
. 2015 Oct 12;5(21):4863-74.
doi: 10.1002/ece3.1736. eCollection 2015 Nov.
Affiliations
- PMID: 26640666
- PMCID: PMC4662304
- DOI: 10.1002/ece3.1736
Warning coloration can be disruptive: aposematic marginal wing patterning in the wood tiger moth
Atsushi Honma et al. Ecol Evol. 2015.
Abstract
Warning (aposematic) and cryptic colorations appear to be mutually incompatible because the primary function of the former is to increase detectability, whereas the function of the latter is to decrease it. Disruptive coloration is a type of crypsis in which the color pattern breaks up the outline of the prey, thus hindering its detection. This delusion can work even when the prey's pattern elements are highly contrasting; thus, it is possible for an animal's coloration to combine both warning and disruptive functions. The coloration of the wood tiger moth (Parasemia plantaginis) is such that the moth is conspicuous when it rests on vegetation, but when it feigns death and drops to the grass- and litter-covered ground, it is hard to detect. This death-feigning behavior therefore immediately switches the function of its coloration from signaling to camouflage. We experimentally tested whether the forewing patterning of wood tiger moths could function as disruptive coloration against certain backgrounds. Using actual forewing patterns of wood tiger moths, we crafted artificial paper moths and placed them on a background image resembling a natural litter and grass background. We manipulated the disruptiveness of the wing pattern so that all (marginal pattern) or none (nonmarginal pattern) of the markings extended to the edge of the wing. Paper moths, each with a hidden palatable food item, were offered to great tits (Parus major) in a large aviary where the birds could search for and attack the "moths" according to their detectability. The results showed that prey items with the disruptive marginal pattern were attacked less often than prey without it. However, the disruptive function was apparent only when the prey was brighter than the background. These results suggest that warning coloration and disruptive coloration can work in concert and that the moth, by feigning death, can switch the function of its coloration from warning to disruptive.
Keywords: Aposematism; camouflage; crypsis; defense; disruptive coloration; predation.
Figures
Figure 1
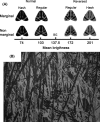
(A) A Parasemia plantaginis individual is highly conspicuous resting on a plant. (B) The same individual feigned death and dropped to the ground when the observer approached it, becoming highly cryptic (the moth is in the center of the photo). (C) Geographic variation in forewing patterns (only right forewings are shown). The Regular pattern (rightmost) is common in central and northern Europe, and the Hash pattern (second from the left) is found mainly in North America.
Figure 2
(A) Mean brightness values of gray tone pixels (range, 0–255) of artificial prey and the background (
BG
) used in the detectability experiment. The eight prey types are Normal marginal Hash, Normal nonmarginal Hash, Normal marginal Regular, Normal nonmarginal Regular, Reversed marginal Regular, Reversed nonmarginal Regular, Reversed marginal Hash, and Reversed nonmarginal Hash. The prey items were created from two natural forewing patterns of P. plantaginis (see Materials and Methods). The brightness of the pale and dark gray patches was 236 and 34, respectively. (B) The background used in the detectability test. A Normal marginal Regular prey item is located near the top and slightly to the right of the centerline.
Figure 3
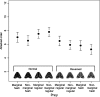
The order in which prey were attacked (mean and 95%
CI
) in the preference test. The prey items are arranged from left to right according to their brightness value. marginal and nonmarginal members of each pair have the same brightness.
Figure 4
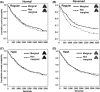
Survival curves of each pair of prey items along the time (sec) from the start of the trial in the detectability test: (A) Normal Regular, (B) Reversed Regular, (C) Normal Hash, and (D) Reversed Hash. The four pairs were analyzed separately because the preference test showed a significant effect of the interaction between prey type and mean prey brightness.
Similar articles
- Disruptive coloration provides camouflage independent of background matching.
Schaefer HM, Stobbe N. Schaefer HM, et al. Proc Biol Sci. 2006 Oct 7;273(1600):2427-32. doi: 10.1098/rspb.2006.3615. Proc Biol Sci. 2006. PMID: 16959631 Free PMC article. - Geographic mosaic of selection by avian predators on hindwing warning colour in a polymorphic aposematic moth.
Rönkä K, Valkonen JK, Nokelainen O, Rojas B, Gordon S, Burdfield-Steel E, Mappes J. Rönkä K, et al. Ecol Lett. 2020 Nov;23(11):1654-1663. doi: 10.1111/ele.13597. Epub 2020 Sep 2. Ecol Lett. 2020. PMID: 32881319 - Disruptive contrast in animal camouflage.
Stevens M, Cuthill IC, Windsor AM, Walker HJ. Stevens M, et al. Proc Biol Sci. 2006 Oct 7;273(1600):2433-8. doi: 10.1098/rspb.2006.3614. Proc Biol Sci. 2006. PMID: 16959632 Free PMC article. - Defining disruptive coloration and distinguishing its functions.
Stevens M, Merilaita S. Stevens M, et al. Philos Trans R Soc Lond B Biol Sci. 2009 Feb 27;364(1516):481-8. doi: 10.1098/rstb.2008.0216. Philos Trans R Soc Lond B Biol Sci. 2009. PMID: 18990673 Free PMC article. Review. - Perspective: the evolution of warning coloration is not paradoxical.
Marples NM, Kelly DJ, Thomas RJ. Marples NM, et al. Evolution. 2005 May;59(5):933-40. Evolution. 2005. PMID: 16136793 Review.
Cited by
- Detectability of a poison frog and its Batesian mimic depends on body posture and viewing angle.
McEwen BL, Yeager J, Kinley I, Anderson HM, Barnett JB. McEwen BL, et al. Behav Ecol. 2024 Oct 4;35(6):arae077. doi: 10.1093/beheco/arae077. eCollection 2024 Nov-Dec. Behav Ecol. 2024. PMID: 39473883 Free PMC article. - Predator selection on phenotypic variability of cryptic and aposematic moths.
Nokelainen O, Silvasti SA, Strauss SY, Wahlberg N, Mappes J. Nokelainen O, et al. Nat Commun. 2024 Feb 23;15(1):1678. doi: 10.1038/s41467-024-45329-5. Nat Commun. 2024. PMID: 38395999 Free PMC article. - Signal detectability and boldness are not the same: the function of defensive coloration in nudibranchs is distance-dependent.
van den Berg CP, Endler JA, Cheney KL. van den Berg CP, et al. Proc Biol Sci. 2023 Jul 26;290(2003):20231160. doi: 10.1098/rspb.2023.1160. Epub 2023 Jul 26. Proc Biol Sci. 2023. PMID: 37491958 Free PMC article. - The evolution of startle displays: a case study in praying mantises.
Vidal-García M, O'Hanlon JC, Svenson GJ, Umbers KDL. Vidal-García M, et al. Proc Biol Sci. 2020 Sep 9;287(1934):20201016. doi: 10.1098/rspb.2020.1016. Epub 2020 Sep 2. Proc Biol Sci. 2020. PMID: 32873210 Free PMC article. - Distance-dependent defensive coloration in the poison frog Dendrobates tinctorius, Dendrobatidae.
Barnett JB, Michalis C, Scott-Samuel NE, Cuthill IC. Barnett JB, et al. Proc Natl Acad Sci U S A. 2018 Jun 19;115(25):6416-6421. doi: 10.1073/pnas.1800826115. Epub 2018 Jun 4. Proc Natl Acad Sci U S A. 2018. PMID: 29866847 Free PMC article.
References
- Abràmoff, M. D. , Magalhäes P. J., and Ram S. J.. 2004. Image processing with image. J. Biophot. Int. 7:36–43.
- Andersson, M. B. 1994. Sexual selection. Princeton Univ. Press, Princeton, NJ.
- Bohlin, T. , Tullberg G. S., and Merilaita S.. 2008. The effect of signal appearance and distance on detection risk in an aposematic butterfly larva (Parnassius apollo). Anim. Behav. 76:577–584.
- Bohlin, T. , Gamberale‐Stille G., Merilaita S., Exnerová A., Stys P., and Tullberg B. S.. 2012. The detectability of the colour pattern in the aposematic firebug, Pyrrhocoris apterus: an image‐based experiment with human ‘predators’. Biol. J. Linn. Soc. 105:806–816.
- Cott, H. B. 1940. Adaptive coloration in animals: their meaning and use. Menthuen & Co., Ltd, London.
LinkOut - more resources
Full Text Sources
Other Literature Sources