Lyophilized allografts without pre-treatment with glutaraldehyde are more suitable than cryopreserved allografts for pulmonary artery reconstruction - PubMed (original) (raw)
Lyophilized allografts without pre-treatment with glutaraldehyde are more suitable than cryopreserved allografts for pulmonary artery reconstruction
J R Olmos-Zúñiga et al. Braz J Med Biol Res. 2016 Feb.
Abstract
Various methods are available for preservation of vascular grafts for pulmonary artery (PA) replacement. Lyophilization and cryopreservation reduce antigenicity and prevent thrombosis and calcification in vascular grafts, so both methods can be used to obtain vascular bioprostheses. We evaluated the hemodynamic, gasometric, imaging, and macroscopic and microscopic findings produced by PA reconstruction with lyophilized (LyoPA) grafts and cryopreserved (CryoPA) grafts in dogs. Eighteen healthy crossbred adult dogs of both sexes weighing between 18 and 20 kg were used and divided into three groups of six: group I, PA section and reanastomosis; group II, PA resection and reconstruction with LyoPA allograft; group III, PA resection and reconstruction with CryoPA allograft. Dogs were evaluated 4 weeks after surgery, and the status of the graft and vascular anastomosis were examined macroscopically and microscopically. No clinical, radiologic, or blood-gas abnormalities were observed during the study. The mean pulmonary artery pressure (MPAP) in group III increased significantly at the end of the study compared with baseline (P=0.02) and final [P=0.007, two-way repeat-measures analysis of variance (RM ANOVA)] values. Pulmonary vascular resistance of groups II and III increased immediately after reperfusion and also at the end of the study compared to baseline. The increase shown by group III vs group I was significant only if compared with after surgery and study end (P=0.016 and P=0.005, respectively, two-way RM ANOVA). Microscopically, permeability was reduced by ≤75% in group III. In conclusion, substitution of PAs with LyoPA grafts is technically feasible and clinically promising.
Figures
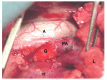
Figure 1. Photograph showing replacement of the left pulmonary artery with a graft. PA: pulmonary artery; A: aorta; G: LyoPA graft; H: heart; L: lung.
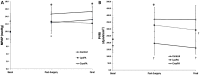
Figure 2. Hemodynamic values for all study groups. Data are reported as means±SD. A, Mean pulmonary artery pressure (MPAP; mmHg, left side): Φ: measurements different from basal value in the CryoPA group (P<0.05); ε: measurements different from basal value in the LyoPA group (P=0.021). B, Pulmonary vascular resistance index (PVRI; dyn/s/cm5), right side): γ: inter-group effects (P=0.004); †: different measurements at the same time between control and CryoPA (P<0.05). Two-way repeated measures ANOVA was used for statistical analyses.
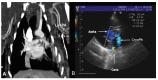
Figure 3. Angiographic and echographic findings at study end. A, lyophilized graft (LyoPA); B, cryopreserved graft (CryoPA). Patency can be observed in the proximal and distal anastomosis of the pulmonary artery graft.
Figure 4. A, Pulmonary artery graft after lyophilization. B, Pulmonary artery graft showing fractures (arrow) after thawing.
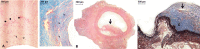
Figure 5. Histologic examination (hematoxylin-eosin staining, ×2.5 magnification) showing: A, control pulmonary artery (arrow: artery wall); B, a lyophilized (LyoPA) graft, pulmonary artery without alterations; and C, a cryopreserved (CryoPA) graft (arrow: artery wall; hi: intimae hyperplasia).
Figure 6. Micrograph of a cryopreserved (CryoPA) graft. A, left. Pulmonary artery with formation of new vessels (thick arrows) and fibroblast proliferation (thin arrows), hematoxylin-eosin, ×10 magnification. A, right. Pulmonary artery with an increased number of collagen fibers (arrows). Masson's trichrome staining, ×10 magnification. B, Artery with a thrombus (arrow) that partially blocked its lumen. Hematoxylin-eosin, ×2 magnification. C, Artery with a thrombus (detail) with area of recanalization (arrow) and collagen deposition (c). Masson's trichrome staining, ×2.5 magnification.
Similar articles
- Pulmonary sleeve resection in locally advanced lung cancer using cryopreserved allograft for pulmonary artery replacement.
Berthet JP, Boada M, Paradela M, Molins L, Matecki S, Marty-Ané CH, Gómez-Caro A. Berthet JP, et al. J Thorac Cardiovasc Surg. 2013 Nov;146(5):1191-7. doi: 10.1016/j.jtcvs.2013.07.003. Epub 2013 Aug 15. J Thorac Cardiovasc Surg. 2013. PMID: 23953718 - Reconstruction of trachea and carina with immediate or cryopreserved allografts in dogs.
Inutsuka K, Kawahara K, Takachi T, Okabayashi K, Shiraishi T, Shirakusa T. Inutsuka K, et al. Ann Thorac Surg. 1996 Nov;62(5):1480-4. doi: 10.1016/0003-4975(96)00473-0. Ann Thorac Surg. 1996. PMID: 8893587 - Cryopreserved arterial allografts used for the treatment of infected vascular grafts.
Desgranges P, Beaujan F, Brunet S, Cavillon A, Qvarfordt P, Mellière D, Becquemin JP. Desgranges P, et al. Ann Vasc Surg. 1998 Nov;12(6):583-8. doi: 10.1007/s100169900204. Ann Vasc Surg. 1998. PMID: 9841690 - Antigenicity of cryopreserved arterial allografts: comparison with fresh and glutaraldehyde treated grafts.
Moriyama S, Utoh J, Sun LB, Tagami H, Okamoto K, Kunitomo R, Kitamura N. Moriyama S, et al. ASAIO J. 2001 May-Jun;47(3):202-5. doi: 10.1097/00002480-200105000-00007. ASAIO J. 2001. PMID: 11374757 - Arterial allografts in treating aortic graft infections: something old, something new.
Vogt PR. Vogt PR. Semin Vasc Surg. 2011 Dec;24(4):227-33. doi: 10.1053/j.semvascsurg.2011.10.008. Semin Vasc Surg. 2011. PMID: 22230678 Review.
Cited by
- Vascular Tissue Engineering: Polymers and Methodologies for Small Caliber Vascular Grafts.
Leal BBJ, Wakabayashi N, Oyama K, Kamiya H, Braghirolli DI, Pranke P. Leal BBJ, et al. Front Cardiovasc Med. 2021 Jan 11;7:592361. doi: 10.3389/fcvm.2020.592361. eCollection 2020. Front Cardiovasc Med. 2021. PMID: 33585576 Free PMC article. Review.
References
- Jasso-Victoria R, Olmos-Zuniga JR, Gutierrez-Marcos LM, Sotres-Vega A, Manjarrez VJ, Gaxiola-Gaxiola M, et al. Usefulness of bovine pericardium as interpositional graft in the surgical repair of nasal septal perforations (experimental study) J Invest Surg. 2003;16:209–217. doi: 10.1080/08941930390214999. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources