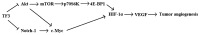Theaflavin-3, 3'-digallate decreases human ovarian carcinoma OVCAR-3 cell-induced angiogenesis via Akt and Notch-1 pathways, not via MAPK pathways - PubMed (original) (raw)
Theaflavin-3, 3'-digallate decreases human ovarian carcinoma OVCAR-3 cell-induced angiogenesis via Akt and Notch-1 pathways, not via MAPK pathways
Ying Gao et al. Int J Oncol. 2016 Jan.
Abstract
Theaflavin-3, 3'-digallate (TF3) is a black tea polyphenol produced from polymerization and oxidization of the green tea ployphenols epicatechin gallate and (-)-epigallocatechin-3-gallate (EGCG) during fermentation of fresh tea leaves. TF3 has been reported to have anticancer properties. However, the effect of TF3 on tumor angiogenesis and the underlying mechanisms are not clear. In the present study, TF3 was verified to inhibit tumor angiogenesis. Compared with EGCG, TF3 was more potent. TF3 inhibited human ovarian carcinoma OVCAR-3 cell-induced angiogenesis in human umbilical vein endothelial cell model and in chick chorioallantoic membrane model. TF3 reduced tumor angiogenesis by downregulating HIF-1α and VEGF. One of the mechanisms was TF3 inactivated Akt/mTOR/p70S6K/4E-BP1 pathway and Akt/c-Myc pathway. Besides, TF3 suppressed the cleavage of Notch-1, subsequently decreased the expression of c-Myc, HIF-1α and VEGF, and finally the impaired cancer cells induced angiogenesis. Nevertheless, TF3 did not have any influence on the MAPK pathways. Taken together, these findings suggest that TF3 might serve as a potential anti-angiogenic agent for cancer treatment.
Figures
Figure 1
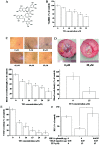
Theaflavin-3, 3′-digallate (TF3) inhibits OVCAR-3 cell-induced angiogenesis by targeting HIF-1α and VEGF. (A) Molecular structure of TF3. (B) Viability of OVCAR-3 cells was decreased after TF3 treatment for 24 h. (C) OVCAR-3 cells-induced HUVEC tube formation was inhibited by TF3 treatment. (D) OVCAR-3 cell-induced blood vessel development in the CAM model was reduced by TF3 treatment. (E) The protein level of VEGF in TF3-treated OVCAR-3 cell culture supernatant was reduced. (F) TF3 diminished the transcriptional activity of VEGF promoter, and, overexpression of HIF-1α reversed it. The data are presented as the mean ± standard error of mean. *P<0.05 compared with control or between specific groups.
Figure 2
Angiogenesis-related proteins are affected by TF3 treatment in OVCAR-3 cells. Western blot analysis revealed that TF3 decreased the protein level of p-Akt, p-mTOR, p-p70S6K, p-4E-BP1, Notch-1 (NICD), c-Myc and HIF-1α in OVCAR-3 cells. TF3 had no impact on the protein level of p-ERK1/2, ERK1/2, JNK, p38 and p-FoxO 1. GAPDH served as the loading control.
Figure 3
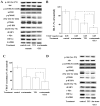
Akt/mTOR/p70S6k/4E-BP1 pathway and Akt/c-Myc pathway are involved in TF3-induced inhibition of HIF-1α and VEGF. (A) Western blot analysis showed that 100 nM wortmannin, 10 μM TF3 and 100 nM wortmannin+10 μM TF3 decreased the phosphorylation of Akt, mTOR, p70S6K and 4E-BP1, and expression of c-Myc and HIF-1α. TF3+wortmannin exhibited the strongest effect among them. GAPDH served as the loading control. (B) Luciferase reporter assay and (C) VEGF ELISA showed 100 nM wortmannin, 10 μM TF3 and 100 nM wortmannin+10 μM TF3 suppressed the transcriptional activity of VEGF promoter and VEGF secretion, respectively. TF3+wortmannin elicited strongest effect among them. (D) Overexpression of active Akt attenuated the 15 μM TF3-induced decrease of phosphorylation of Akt, mTOR, p70S6K and 4E-BP1, and expression of c-Myc and HIF-1α. Overexpression of Akt, mTOR, p70S6K or 4E-BP1 attenuated TF3-induced inhibition of transcriptional activity of VEGF promoter (E) and HIF-1α promoter (F). (G) Overexpression of active Akt reversed the 15 μM TF3-induced reduction of VEGF secretion. The data are presented as the mean ± standard error of mean. *P<0.05 compared with control or between specific groups.
Figure 3
Akt/mTOR/p70S6k/4E-BP1 pathway and Akt/c-Myc pathway are involved in TF3-induced inhibition of HIF-1α and VEGF. (A) Western blot analysis showed that 100 nM wortmannin, 10 μM TF3 and 100 nM wortmannin+10 μM TF3 decreased the phosphorylation of Akt, mTOR, p70S6K and 4E-BP1, and expression of c-Myc and HIF-1α. TF3+wortmannin exhibited the strongest effect among them. GAPDH served as the loading control. (B) Luciferase reporter assay and (C) VEGF ELISA showed 100 nM wortmannin, 10 μM TF3 and 100 nM wortmannin+10 μM TF3 suppressed the transcriptional activity of VEGF promoter and VEGF secretion, respectively. TF3+wortmannin elicited strongest effect among them. (D) Overexpression of active Akt attenuated the 15 μM TF3-induced decrease of phosphorylation of Akt, mTOR, p70S6K and 4E-BP1, and expression of c-Myc and HIF-1α. Overexpression of Akt, mTOR, p70S6K or 4E-BP1 attenuated TF3-induced inhibition of transcriptional activity of VEGF promoter (E) and HIF-1α promoter (F). (G) Overexpression of active Akt reversed the 15 μM TF3-induced reduction of VEGF secretion. The data are presented as the mean ± standard error of mean. *P<0.05 compared with control or between specific groups.
Figure 4
Notch-1/c-Myc pathway is related to TF3-induced inhibition of HIF-1α and VEGF. (A) Western blot analysis showed that 10 μM TF3, 75 μM DAPT and 10 μM TF3+75 μM DAPT decreased the expression of NICD, c-Myc and HIF-1α. The dose of 10 μM TF3+75 μM DAPT exhibited the strongest effect. GAPDH served as the loading control. (B) Luciferase reporter assay and (C) VEGF ELISA showed 10 μM TF3, 75 μM DAPT and 10 μM TF3+75 μM DAPT suppressed the transcriptional activity of VEGF promoter and VEGF secretion, respectively. (D) Overexpression of NICD attenuated 15 μM TF3-induced decrease of NICD, c-Myc and HIF-1α. (E) Overexpression of NICD or c-Myc attenuated TF3-induced inhibition of transcriptional activity of VEGF promoter and HIF-1α promoter. (F) Overexpression of NICD or active Akt attenuated TF3-induced inhibition of transcriptional activity of c-Myc promoter. (G) Overexpression of NICD reversed the 15 μM TF3-induced reduction of VEGF secretion. The data are presented as the mean ± standard error of mean. *P<0.05 compared with control or between specific groups.
Figure 5
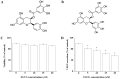
EGCG decreases VEGF secretion of OVCAR-3 cells. (A) Chemical structure of epicatechin gallate. (B) Chemical structure of EGCG. (C) Viability and (D) VEGF secretion of EGCG-treated OVCAR-3 cells. All data are presented as the mean ± standard error of mean. *P<0.05 compared with the control group.
Figure 6
Proposed mechanism of inhibition of tumor angiogenesis via Akt and Notch-1 pathways.
Similar articles
- Theaflavin-3,3'-Digallate Suppresses Human Ovarian Carcinoma OVCAR-3 Cells by Regulating the Checkpoint Kinase 2 and p27 kip1 Pathways.
Gao Y, Yin J, Tu Y, Chen YC. Gao Y, et al. Molecules. 2019 Feb 14;24(4):673. doi: 10.3390/molecules24040673. Molecules. 2019. PMID: 30769778 Free PMC article. - The epigallocatechin gallate derivative Y6 inhibits human hepatocellular carcinoma by inhibiting angiogenesis in MAPK/ERK1/2 and PI3K/AKT/ HIF-1α/VEGF dependent pathways.
Liao ZH, Zhu HQ, Chen YY, Chen RL, Fu LX, Li L, Zhou H, Zhou JL, Liang G. Liao ZH, et al. J Ethnopharmacol. 2020 Sep 15;259:112852. doi: 10.1016/j.jep.2020.112852. Epub 2020 Apr 9. J Ethnopharmacol. 2020. PMID: 32278759 - Theaflavin-3, 3'-digallate induces apoptosis and G2 cell cycle arrest through the Akt/MDM2/p53 pathway in cisplatin-resistant ovarian cancer A2780/CP70 cells.
Tu Y, Kim E, Gao Y, Rankin GO, Li B, Chen YC. Tu Y, et al. Int J Oncol. 2016 Jun;48(6):2657-65. doi: 10.3892/ijo.2016.3472. Epub 2016 Apr 6. Int J Oncol. 2016. PMID: 27082635 Free PMC article. - Theasaponin E1 Inhibits Platinum-Resistant Ovarian Cancer Cells through Activating Apoptosis and Suppressing Angiogenesis.
Li B, Tong T, Ren N, Rankin GO, Rojanasakul Y, Tu Y, Chen YC. Li B, et al. Molecules. 2021 Mar 17;26(6):1681. doi: 10.3390/molecules26061681. Molecules. 2021. PMID: 33802884 Free PMC article.
Cited by
- The role of nanomaterials in enhancing natural product translational potential and modulating endoplasmic reticulum stress in the treatment of ovarian cancer.
Singla RK, Sharma P, Kumar D, Gautam RK, Goyal R, Tsagkaris C, Dubey AK, Bansal H, Sharma R, Shen B. Singla RK, et al. Front Pharmacol. 2022 Oct 26;13:987088. doi: 10.3389/fphar.2022.987088. eCollection 2022. Front Pharmacol. 2022. PMID: 36386196 Free PMC article. Review. - In vivo antiangiogenic effect of nimbolide, trans-chalcone and piperine for use against glioblastoma.
Senrung A, Tripathi T, Yadav J, Janjua D, Chaudhary A, Chhokar A, Aggarwal N, Joshi U, Goswami N, Bharti AC. Senrung A, et al. BMC Cancer. 2023 Nov 30;23(1):1173. doi: 10.1186/s12885-023-11625-4. BMC Cancer. 2023. PMID: 38036978 Free PMC article. - Association between Different Types of Tea Consumption and Risk of Gynecologic Cancer: A Meta-Analysis of Cohort Studies.
Zheng F, Chen K, Zhong J, Tang S, Xu S, Lu W, Wu Y, Xia D. Zheng F, et al. Nutrients. 2023 Jan 13;15(2):403. doi: 10.3390/nu15020403. Nutrients. 2023. PMID: 36678274 Free PMC article. - Modulation of Notch Signaling Pathway by Bioactive Dietary Agents.
Kiesel VA, Stan SD. Kiesel VA, et al. Int J Mol Sci. 2022 Mar 24;23(7):3532. doi: 10.3390/ijms23073532. Int J Mol Sci. 2022. PMID: 35408894 Free PMC article. Review. - Targeting of Lung Cancer Stem Cell Self-Renewal Pathway by a Small Molecule Verrucarin J.
Udoh K, Parte S, Carter K, Mack A, Kakar SS. Udoh K, et al. Stem Cell Rev Rep. 2019 Aug;15(4):601-611. doi: 10.1007/s12015-019-09874-7. Stem Cell Rev Rep. 2019. PMID: 30835047 Free PMC article.
References
- Mizuno T, Suzuki N, Makino H, Furui T, Morii E, Aoki H, Kunisada T, Yano M, Kuji S, Hirashima Y, et al. Cancer stem-like cells of ovarian clear cell carcinoma are enriched in the ALDH-high population associated with an accelerated scavenging system in reactive oxygen species. Gynecol Oncol. 2015;137:299–305. doi: 10.1016/j.ygyno.2014.12.005. - DOI - PubMed
- Barakat RR, Markman M, Randall M. Principles and Practice of Gynecologic Oncology. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia, PA: 2009.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous