A Small Molecule that Induces Intrinsic Pathway Apoptosis with Unparalleled Speed - PubMed (original) (raw)
A Small Molecule that Induces Intrinsic Pathway Apoptosis with Unparalleled Speed
Rahul Palchaudhuri et al. Cell Rep. 2015.
Abstract
Apoptosis is generally believed to be a process that requires several hours, in contrast to non-programmed forms of cell death that can occur in minutes. Our findings challenge the time-consuming nature of apoptosis as we describe the discovery and characterization of a small molecule, named Raptinal, which initiates intrinsic pathway caspase-dependent apoptosis within minutes in multiple cell lines. Comparison to a mechanistically diverse panel of apoptotic stimuli reveals that Raptinal-induced apoptosis proceeds with unparalleled speed. The rapid phenotype enabled identification of the critical roles of mitochondrial voltage-dependent anion channel function, mitochondrial membrane potential/coupled respiration, and mitochondrial complex I, III, and IV function for apoptosis induction. Use of Raptinal in whole organisms demonstrates its utility for studying apoptosis in vivo for a variety of applications. Overall, rapid inducers of apoptosis are powerful tools that will be used in a variety of settings to generate further insight into the apoptotic machinery.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
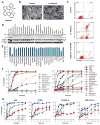
Figure 1. Raptinal Rapidly Induces Apoptosis
A) Structure of Raptinal. B) Scanning electron micrographs of U-937 cells show pronounced apoptotic blebbing after 1 hour of treatment with 10 μM Raptinal (right) versus vehicle control treated cells (left). C) AV/PI graphs of U-937 cells treated with 10 μM Raptinal for 2 hours show transition of cells through the early apoptotic AV+/PI− quadrant that is prevented by the pan-caspase inhibitor Q-VD-OPh. D) Immunoblots of U-937 cells treated with various toxins for 1 hour show more prominent activation of procaspase-3 (PC-3) to caspase-3 (C-3) and cleavage of PARP-1 (cPARP-1) by Raptinal (10 μM) versus 25 other toxins (all tested at 10 μM). E) Cell viability of U-937 cells assessed by AV/PI after 2 hour treatment with 10 μM Raptinal and 25 other small molecules (all tested at 10 μM). Data represent the mean ± SD from 3 independent experiments. F) Time course analysis of U-937 cell viability upon treatment with 10 μM of various anticancer agents and biological tool molecules. Cell viability was assessed by AV/PI analysis. G) Time course analysis of adherent cell viability upon treatment with Raptinal, 1541B, and Staurosporine (all tested at 10 μM). Cell viability was assessed by AV/PI analysis. See also Figure S1.
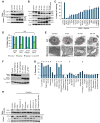
Figure 2. Raptinal Activates the Intrinsic Pathway and Requires Functional Mitochondria for Apoptosis Induction
A) Time course immunoblots of mitochondrial and cytosolic fractions of U-937 cells treated with 10 μM Raptinal show cytochrome c release and subsequent caspase-9 activation occur after 20 minutes of treatment. B) Time course immunoblot analysis of caspase-9, -3, and -8 activation in U-937 cells treated with 10 μM Raptinal. C) Relative percent caspase-3/-7 activity of MIA PaCa-2 cells treated with 10 μM Raptinal for 1 hour upon siRNA knockdown of apoptosis genes. D) Jurkat C8−/− are equally susceptible, Jurkat FADD−/− are more susceptible, while Jurkat Bcl-2 overexpressing cells are less susceptible than wild type cells to 10 μM Raptinal as assessed by AV/PI assay after 2 hours. Data represent the mean ± SD from 3 independent experiments. ** Indicates p values < 0.02 E) Transmission electron micrographs of U-937 cells treated with vehicle or 10 μM Raptinal for 5, 30 and 60 minutes. The images show rapid changes in mitochondrial morphology (arrows) after 5 minutes of treatment with Raptinal. At 30 minutes, mitochondria are largely devoid of cristae and at 60 minutes, peripheral nuclear condensation is apparent. Scale bars represent 1 micron. F) Raptinal does not induce cytochrome c release from the mitochondrial pellet into the supernatant of isolated mitochondria treated with Raptinal in vitro under non-respiring conditions. The positive control, pro-apoptotic Bid protein is able to induce cytochrome c release. G) U-937 cells pre-treated with various potential cytoprotective agents and inhibitors of various cellular pathways were treated with 10 μM Raptinal for 2 hours and protection from the effects of Raptinal was assessed by AV/PI. Data represent the mean % protection ± SD from 3 independent experiments. * Indicates p values < 0.05. H) Protective mitochondrial agents retard cytochrome c release and caspase 9 activation in U-937 cells. See also Figure S2.
Figure 3. Raptinal Exhibits Activity in vivo
A) Zebrafish embryos expressing secretory annexin V-YFP exhibit pronounced punctate YFP signal indicating phosphatidylserine externalization following 1.5 hours of treatment with 10 μM Raptinal. B) Quantification of apoptotic cells in Raptinal- versus DMSO-treated zebrafish under the conditions in A. Data represent the mean ± SD (n = 5 and n = 7 embryos for Raptinal and DMSO, respectively; *** indicates p value < 0.001). Raptinal inhibits subcutaneous B16-F10 melanoma tumor growth in vivo as measured by tumor volume (** indicates p value < 0.005) in C and tumor mass after tumor excision in D (* indicates p value < 0.05). E–F) Raptinal inhibits subcutaneous 4T1 breast cancer tumor growth in vivo as measured by tumor volume (* indicates p value < 0.05) in E and tumor mass after tumor excision (* indicates p value < 0.05) in F. Arrows in C and E indicate intraperitoneal Raptinal administration at 20 mg/kg once a day. Tumor images in D and F are representative of tumor size at the conclusion of the studies. Data in C, D, E and F represent the mean ± SEM (n = 7 mice/group). See also Figure S3.
Similar articles
- Critical role of the death receptor pathway in the antitumoral effects induced by hispanolone derivatives.
Través PG, López-Fontal R, Cuadrado I, Luque A, Boscá L, de las Heras B, Hortelano S. Través PG, et al. Oncogene. 2013 Jan 10;32(2):259-68. doi: 10.1038/onc.2012.23. Epub 2012 Feb 6. Oncogene. 2013. PMID: 22310289 - The small molecule raptinal can simultaneously induce apoptosis and inhibit PANX1 activity.
Santavanond JP, Chiu YH, Tixeira R, Liu Z, Yap JKY, Chen KW, Li CL, Lu YR, Roncero-Carol J, Hoijman E, Rutter SF, Shi B, Ryan GF, Hodge AL, Caruso S, Baxter AA, Ozkocak DC, Johnson C, Day ZI, Mayfosh AJ, Hulett MD, Phan TK, Atkin-Smith GK, Poon IKH. Santavanond JP, et al. Cell Death Dis. 2024 Feb 9;15(2):123. doi: 10.1038/s41419-024-06513-z. Cell Death Dis. 2024. PMID: 38336804 Free PMC article. - The mitochondrial voltage-dependent anion channel 1 in tumor cells.
Shoshan-Barmatz V, Ben-Hail D, Admoni L, Krelin Y, Tripathi SS. Shoshan-Barmatz V, et al. Biochim Biophys Acta. 2015 Oct;1848(10 Pt B):2547-75. doi: 10.1016/j.bbamem.2014.10.040. Epub 2014 Nov 4. Biochim Biophys Acta. 2015. PMID: 25448878 Review. - Raptinal: a powerful tool for rapid induction of apoptotic cell death.
Smith AJ, Hergenrother PJ. Smith AJ, et al. Cell Death Discov. 2024 Aug 21;10(1):371. doi: 10.1038/s41420-024-02120-1. Cell Death Discov. 2024. PMID: 39164225 Free PMC article. Review.
Cited by
- Mitochondria-targeted senotherapeutic interventions.
Atayik MC, Çakatay U. Atayik MC, et al. Biogerontology. 2022 Aug;23(4):401-423. doi: 10.1007/s10522-022-09973-y. Epub 2022 Jul 4. Biogerontology. 2022. PMID: 35781579 Review. - cFLIPS regulates alternative NLRP3 inflammasome activation in human monocytes.
Gao Y, Yu S, Chen M, Wang X, Pan L, Wei B, Meng G. Gao Y, et al. Cell Mol Immunol. 2023 Oct;20(10):1203-1215. doi: 10.1038/s41423-023-01077-y. Epub 2023 Aug 17. Cell Mol Immunol. 2023. PMID: 37591930 Free PMC article. - Characterization and identification of cell death dynamics by quantitative phase imaging.
Hsieh HC, Lin PT, Sung KB. Hsieh HC, et al. J Biomed Opt. 2022 Apr;27(4):046502. doi: 10.1117/1.JBO.27.4.046502. J Biomed Opt. 2022. PMID: 35484694 Free PMC article. - Induction of Apoptosis and Inhibition of Invasion in Gastric Cancer Cells by Titanium Dioxide Nanoparticles.
Nasr R, Hasanzadeh H, Khaleghian A, Moshtaghian A, Emadi A, Moshfegh S. Nasr R, et al. Oman Med J. 2018 Mar;33(2):111-117. doi: 10.5001/omj.2018.22. Oman Med J. 2018. PMID: 29657679 Free PMC article. - Tricking a Cancer Cell into Committing Suicide or Apoptosis.
Chintamani. Chintamani. Indian J Surg. 2017 Feb;79(1):4-5. doi: 10.1007/s12262-017-1598-y. Epub 2017 Jan 28. Indian J Surg. 2017. PMID: 28331258 Free PMC article. No abstract available.
References
- Bair JS, Palchaudhuri R, Hergenrother PJ. Chemistry and biology of deoxynyboquinone, a potent inducer of cancer cell death. J Am Chem Soc. 2010;132:5469–5478. - PubMed
- Balasubramanian K, Mirnikjoo B, Schroit AJ. Regulated externalization of phosphatidylserine at the cell surface: implications for apoptosis. J Biol Chem. 2007;282:18357–18364. - PubMed
- Bosserman L, Prendergast F, Herbst R, Fleisher M, Salom E, Strickland S, Raptis A, Hallquist A, Perree M, Rajurkar S, et al. The microculture-kinetic (MiCK) assay: the role of a drug-induced apoptosis assay in drug development and clinical care. Cancer Res. 2012;72:3901–3905. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1-T32-GM070421/GM/NIGMS NIH HHS/United States
- R01 CA120439/CA/NCI NIH HHS/United States
- T32 GM070421/GM/NIGMS NIH HHS/United States
- U54 NS079201/NS/NINDS NIH HHS/United States
- R01-CA120439/CA/NCI NIH HHS/United States
- NS079201/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases