IL-27 induces the expression of IDO and PD-L1 in human cancer cells - PubMed (original) (raw)
IL-27 induces the expression of IDO and PD-L1 in human cancer cells
Grazia Carbotti et al. Oncotarget. 2015.
Abstract
IL-27 is a member of the IL-12 family that is produced by macrophages and dendritic cells. IL-27 inhibits the growth and invasiveness of different cancers and therefore represents a potential anti-tumor agent. By contrast, it may exert immune-regulatory properties in different biological systems. We reported that IL-27 induces the expression of the IL-18 inhibitor IL-18BP, in human Epithelial Ovarian Cancer (EOC) cells, thus potentially limiting the immune response. Here, we tested whether IL-27 may modulate other immune-regulatory molecules involved in EOC progression, including Indoleamine 2,3-dioxygenase (IDO) and Programmed Death-Ligand (PD-L)1. IDO and PD-L1 were not constitutively expressed by EOC cells in vitro, but IL-27 increased their expression through STAT1 and STAT3 tyrosine phosphorylation. Differently, cells isolated from EOC ascites showed constitutive activation of STAT1 and STAT3 and IDO expression. These findings, together with the expression of IL-27 in scattered leukocytes in EOC ascites and tissues, suggest a potential role of IL-27 in immune-regulatory networks of EOC. In addition, IL-27 induced IDO or PD-L1 expression in monocytes and in human PC3 prostate and A549 lung cancer cells. A current paradigm in tumor immunology is that tumor cells may escape from immune control due to "adaptive resistance" mediated by T cell-secreted IFN-γ, which induces PD-L1 and IDO expression in tumor cells. Our present data indicate that also IL-27 has similar activities and suggest that the therapeutic use of IL-27 as anti-cancer agent may have dual effects, in some tumors.
Keywords: IDO; IL-27; Immune response; Immunity; Immunology and Microbiology Section; PD-L1; STAT.
Conflict of interest statement
CONFLICTS OF INTEREST
The authors have no conflict of interest to disclose.
Figures
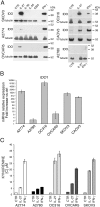
Figure 1. IL-27 induces IDO protein and mRNA expression in human EOC cells in vitro
A. Western blot analysis of IDO expression in six EOC cell lines stimulated with the indicated cytokines or medium only (CTR) for 48 hours. α-tubulin is used as loading control. A representative experiment out of two is shown. B. QRT-PCR analysis of IDO1 mRNA expression in EOC cells treated with IL-27 relative to untreated (Ctrl) cells. Data are the mean of three independent experiments and expressed as ΔΔCT-fold change. Error bars represent SD. C. Kynurenine production in the conditioned medium of IL-27, IFN-γ or untreated (CTR) EOC cells, as detected by HPLC analysis. Histograms represent mean values of three biological replicates and error bars are standard deviations.
Figure 2. IL-27 increases PD-L1 surface protein and mRNA expression in EOC cells in vitro
A. FACS analysis of surface PD-L1 expression in four EOC cell lines, cultured in the presence of medium (control), IL-27 or IFN-γ. Dotted lines are isotype-matched unrelated Ig staining controls. Numbers in brackets are Median Fluorescence Intensity (MFI) values calculated as median PD-L1 minus median Ig control. Data are representative of two independent experiments showing similar results. B. QRT-PCR analysis of PDL1 mRNA expression in five IL-27-stimulated EOC cells relative to untreated cells. Data are the mean (±SD) of three independent experiments. C. Comparative analysis of IDO1 and PDL1 mRNA up-regulation by IL-27 or IFN-γ in a representative EOC cell line (CAOV3). Data are the mean of two independent replicates and are expressed as ΔΔCT-fold change. Error bars represent the minimum and maximum.
Figure 3. IL-27R chains GP130 and WSX1/IL-27RA are expressed in EOC cell lines
FACS analysis of surface GP130 and WSX1/IL-27RA expression in six EOC cell lines. Dotted lines represent isotype-matched unrelated Ig staining controls. Numbers in brackets are MFI values calculated as median GP130 or WSX1/IL-27RA minus median Ig control. Data are representative of two independent experiments with similar results.
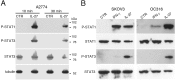
Figure 4. IL-27 induces STAT1 and STAT3 phosphorylation (P) in EOC cell lines in vitro
A. Western blot analysis of tyrosine phosphorylated (P)-STAT1, P-STAT3 and STAT3 proteins in A2774 EOC cells either cultured for 10 or 30 minutes with medium (CTR) or with IL-27 (20 ng/ml). Total STAT3 and α-tubulin served as controls. Similar kinetics of STAT1/3 phosphorylation were observed in another cell line (A2780, not shown). B. Analysis of P-STAT1, STAT1, P-STAT3 and STAT3 proteins in SKOV3 and OC316 cell lines cultured in medium alone (CTR), or with IFN-γ or IL-27 (20 minutes). Total STAT1 and STAT3 were used as controls. Data are representative of three independent experiments.
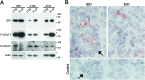
Figure 5. IDO and tyrosine-phosphorylated forms of STAT1 and STAT3 are constitutively present in cells from EOC ascites
A. Western blot analysis of IDO, P-STAT1 and P-STAT3 proteins in neoplastic cells isolated from the ascites of three ovarian cancer patients cultured with medium (CTR) or IL-27 for 20 minutes. β-actin was used as loading control. * indicate lanes from a blot re-probed for IDO and phosphorylated STAT3 for which STAT1 and actin were presented in a previous article [17]. B. Immunohistochemical analysis of IDO expression in ascites cells. Both tumor cell nests (arrow) and scattered reactive cells express IDO protein.
Figure 6. Silencing of STAT1 or STAT3 with siRNA effects IL-27-driven IDO or PD-L1 expression
A. Western blot analysis of STAT1 and STAT3 unphosphorylated and phosphorylated (P) proteins in STAT1- or STAT3-silenced or scrambled (NT) siRNA-transfected A2774 and OC316 EOC cells untreated or treated for 20 minutes with IL-27 (20 ng/ml). Total STAT3 or STAT1 served as controls for STAT1- or STAT3-silenced cells, respectively. B. Western blot analysis of IDO expression in STAT1- or STAT3-silenced or scrambled siRNA-transfected A2774 and OC316 EOC cells untreated or treated for 48 hours with IL-27 (50 ng/ml). α-tubulin is shown as loading control. A replicate experiment is shown in Figure S6A C. FACS analysis of PD-L1 surface expression in STAT1- or STAT3-silenced or scrambled siRNA-transfected OC316 or A2774 EOC cells untreated or treated for 48 hours with IL-27 (50 ng/ml). Numbers in brackets are MFI values calculated as median PD-L1 minus median Ig control. The results of different experiments are shown in Figure S6B.
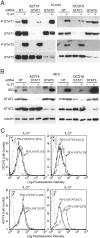
Figure 7. IL-27 induces PD-L1 and/or IDO expression in human PC3 prostate and A549 lung cancer cells and adherent PBMC
A. Western blot analysis of IDO expression in human adherent PBMC, non-adherent PBMC and in PC3 and A549 cells treated with the indicated cytokines or medium only for 48 hours. α-tubulin is used as loading control. Similar results were observed in two additional experiments. B. QRT-PCR analysis of IDO1 and PDL1 mRNA expression in cytokine-stimulated adherent or non-adherent PBMC, PC3 and A549 cells relative to untreated cells. Data are expressed as ΔΔCT-fold change. Mean values of three independent experiments. Error bars represent SD. C. FACS analysis of surface PD-L1 in IL-27-treated or untreated PC3 and A549 cell lines and adherent PBMC. Dotted lines are isotype-matched unrelated Ig staining controls. Similar results were obtained in five different experiments (PC3: MFI 5.5 ± 2.7 vs 1.5 ± 0.6, mean ± SD in IL-27-treated vs un-stimulated cells, P = 0.03 by paired Student's t test; A549: MFI 2 ± 1 vs 0.43 ± 0.4, P = 0.01).
Similar articles
- Targetable Immune Regulatory Molecule Expression in High-Grade Serous Ovarian Carcinomas in African American Women: A Study of PD-L1 and IDO in 112 Cases From the African American Cancer Epidemiology Study (AACES).
Mills AM, Peres LC, Meiss A, Ring KL, Modesitt SC, Abbott SE, Alberg AJ, Bandera EV, Barnholtz-Sloan J, Bondy ML, Cote ML, Funkhouser E, Moorman PG, Peters ES, Schwartz AG, Terry PD, Wallace K, Schildkraut JM. Mills AM, et al. Int J Gynecol Pathol. 2019 Mar;38(2):157-170. doi: 10.1097/PGP.0000000000000494. Int J Gynecol Pathol. 2019. PMID: 29485423 Free PMC article. - Triple blockade of Ido-1, PD-L1 and MEK as a potential therapeutic strategy in NSCLC.
Della Corte CM, Ciaramella V, Ramkumar K, Vicidomini G, Fiorelli A, Nardone V, Cappabianca S, Cozzolino I, Zito Marino F, Di Guida G, Wang Q, Cardnell R, Gay CM, Ciardiello D, Martinelli E, Troiani T, Martini G, Napolitano S, Wang J, Byers LA, Ciardiello F, Morgillo F. Della Corte CM, et al. J Transl Med. 2022 Nov 22;20(1):541. doi: 10.1186/s12967-022-03730-y. J Transl Med. 2022. PMID: 36419183 Free PMC article. - Clinical Significance of Program Death Ligand-1 and Indoleamine-2,3-Dioxygenase Expression in Colorectal Carcinoma.
Hacking S, Vitkovski T, Jain S, Jin C, Chavarria H, Wu D, Nasim M. Hacking S, et al. Appl Immunohistochem Mol Morphol. 2021 Mar 1;29(3):201-208. doi: 10.1097/PAI.0000000000000868. Appl Immunohistochem Mol Morphol. 2021. PMID: 32842025 - Dual inhibition of STAT1 and STAT3 activation downregulates expression of PD-L1 in human breast cancer cells.
Sasidharan Nair V, Toor SM, Ali BR, Elkord E. Sasidharan Nair V, et al. Expert Opin Ther Targets. 2018 Jun;22(6):547-557. doi: 10.1080/14728222.2018.1471137. Epub 2018 May 2. Expert Opin Ther Targets. 2018. PMID: 29702007 Review. - The Balance Players of the Adaptive Immune System.
Andersen MH. Andersen MH. Cancer Res. 2018 Mar 15;78(6):1379-1382. doi: 10.1158/0008-5472.CAN-17-3607. Epub 2018 Feb 13. Cancer Res. 2018. PMID: 29440147 Review.
Cited by
- Mechanisms regulating PD-L1 expression in cancers and associated opportunities for novel small-molecule therapeutics.
Yamaguchi H, Hsu JM, Yang WH, Hung MC. Yamaguchi H, et al. Nat Rev Clin Oncol. 2022 May;19(5):287-305. doi: 10.1038/s41571-022-00601-9. Epub 2022 Feb 7. Nat Rev Clin Oncol. 2022. PMID: 35132224 Review. - Extracellular vesicle PD-L1 in reshaping tumor immune microenvironment: biological function and potential therapy strategies.
Liu J, Peng X, Yang S, Li X, Huang M, Wei S, Zhang S, He G, Zheng H, Fan Q, Yang L, Li H. Liu J, et al. Cell Commun Signal. 2022 Jan 28;20(1):14. doi: 10.1186/s12964-021-00816-w. Cell Commun Signal. 2022. PMID: 35090497 Free PMC article. Review. - Intravenous administration of LPS activates the kynurenine pathway in healthy male human subjects: a prospective placebo-controlled cross-over trial.
Millischer V, Heinzl M, Faka A, Resl M, Trepci A, Klammer C, Egger M, Dieplinger B, Clodi M, Schwieler L. Millischer V, et al. J Neuroinflammation. 2021 Jul 17;18(1):158. doi: 10.1186/s12974-021-02196-x. J Neuroinflammation. 2021. PMID: 34273987 Free PMC article. Clinical Trial. - Regulatory mechanisms of PD-L1 expression in cancer cells.
Shi Y. Shi Y. Cancer Immunol Immunother. 2018 Oct;67(10):1481-1489. doi: 10.1007/s00262-018-2226-9. Epub 2018 Aug 17. Cancer Immunol Immunother. 2018. PMID: 30120503 Free PMC article. Review. - NCoR1: Putting the Brakes on the Dendritic Cell Immune Tolerance.
Ahad A, Stevanin M, Smita S, Mishra GP, Gupta D, Waszak S, Sarkar UA, Basak S, Gupta B, Acha-Orbea H, Raghav SK. Ahad A, et al. iScience. 2019 Sep 27;19:996-1011. doi: 10.1016/j.isci.2019.08.024. Epub 2019 Aug 17. iScience. 2019. PMID: 31522122 Free PMC article.
References
- Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nature medicine. 2003;9:1269–1274. - PubMed
- Munn DH. Indoleamine 2,3-dioxygenase, Tregs and cancer. Current medicinal chemistry. 2011;18:2240–2246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous