The positive prognostic effect of stromal CD8+ tumor-infiltrating T cells is restrained by the expression of HLA-E in non-small cell lung carcinoma - PubMed (original) (raw)
The positive prognostic effect of stromal CD8+ tumor-infiltrating T cells is restrained by the expression of HLA-E in non-small cell lung carcinoma
Mehrdad Talebian Yazdi et al. Oncotarget. 2016.
Abstract
Introduction: Tumor-infiltrating CD8+ T cells are associated with improved clinical outcomes in non-small cell lung cancer (NSCLC). Here we studied their prognostic effect in the context of the expression of HLA molecules that are key in tumor recognition (HLA-A, B and C) or suppression of immunity (HLA-E) as this is still unknown.
Methods: Tumor tissue of 197 patients with resected pulmonary adenocarcinoma was analyzed for the presence of CD8+ T cells and the expression of β2-microglobulin, HLA-A, HLA-B/C and HLA-E. The relation of these parameters with overall survival (OS) was assessed.
Results: Loss and low expression of HLA-A or HLA-B/C was found in 44% and 75% of cases respectively. A high CD8+ tumor infiltration was strongly associated with clinical benefit only when the tumors retained good expression of HLA-A and HLA-B/C (p=0.004). In addition, more than 70% of the tumors were found to display a high expression of HLA-E. The expression of HLA-E by tumor cells was an independent negative prognostic factor for OS (p=0.031). Importantly, a dense stromal CD8+ T cell infiltration was strongly associated with improved OS only in HLA-E negative tumors (p=0.005) and its prognostic effect was completely abolished when tumors highly expressed HLA-E (p=0.989).
Conclusions: CD8+ T cell infiltration strongly contributes to a better prognosis in NSCLC when the tumor cells retain the expression of classical HLA class I and do not express HLA-E. Therefore, analysis of HLA-A, -B/C and HLA-E expression should be included as biomarkers to predict the response to immunotherapy.
Keywords: CD8+ T cells; HLA class I; HLA-E; non-small cell lung cancer; survival.
Conflict of interest statement
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Figures
Figure 1. Staining of tumor infiltrating CD8+ T cells, β2-microglobulin, HLA-A, HLA-B/C and HLA-E in pulmonary adenocarcinoma
Formalin-fixed, paraffin embedded tumor specimens of 197 non-small cell lung cancer patients were cut in 4 μm sections and immunohistochemically stained for CD8, β2-microglobulin, classical HLA-A and HLA-B/C, as well as non-classical HLA-E. According to the Ruiter scoring system [46] both the intensity and percentage of cells stained were assessed and expression was categorized as low (score 1-4) and high (score 5-9). Examples are shown of high A. and low B. stromal and intraepithelial CD8+ T cell infiltration; tumor with high β2-microglobulin expression C. examples of HLA-A D. HLA-B/C E. and HLA-E F. staining. Original magnification x200.
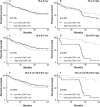
Figure 2. Association of CD8+ T cell infiltration and HLA expression with overall survival (OS)
Patients were divided in two groups with low or high CD8+ T cell infiltration, based on the mean CD8+ T-cell count for all patients or on the basis of HLA expression. OS was defined as date of surgery until date of death due to any cause, or date of last follow-up with a maximum follow-up time of 5 years. Kaplan-Meier curves were used to estimate OS of the two groups whereas the log-rank test was used to compare the difference between the two curves. Survival curves are presented for A. patients with low (n=104) or high (n=59) intraepithelial CD8+ T cells (mean CD8+ cell count 194 cells/mm2 tumor) ; B. low (n=92) or high (n=71) stromal CD8+ T cells (mean CD8+ cell count 348 cells/mm2 tumor) ; C. low (n=95) or high (n=68) total CD8+ T cells (mean CD8+ cell count 271 cells/mm2 tumor). Furthermore, survival curves are presented for functional (i.e. positive staining for both HLA and β2-M) expression of D. HLA-A low (n=106) and high (n=91) ; E. HLA-B/C low (n=156) and high (n=41) ; F. HLA-E low (n=87) and high (n=110). A significant correlation (p=0.042) was observed between low HLA-E expression and improved survival (F).
Figure 3. Effect of classical HLA class I expression and CD8+ T cell infiltration on overall survival (OS)
Patients were divided in two groups with low or high CD8+ T cell infiltration, based on the mean CD8+ T-cell count for all patients and on the expression of classical HLA class I. Kaplan-Meier curves were constructed to estimate OS of the two groups whereas the log-rank test was used to compare the difference between the two curves. A, B. Comparison of OS between patients with low or high total CD8+ T cell infiltration in the context of low (A; n=63 vs 30, respectively) or high (B; n=32 vs 38, respectively) HLA-A expression. C, D. Comparison of OS between patients with low (n=83) or high (n=47) CD8+ T cell infiltration of whom the tumors displayed low HLA-B/C expression (C). Comparison of OS between patients with HLA-B/C positive tumors and high (n=21) or low (n=12) total CD8+ T cell infiltration (D). E, F. Comparison of OS between patients with low (n=62) or high (n=27) CD8+ T cell infiltration in tumors with low expression of both HLA-A and HLA-B/C (E). Comparison of OS between patients with low (n=11) or high (n=18) total CD8+ T cell infiltration in the context of tumors with high expression of both HLA-A and HLA-B/C. (F)
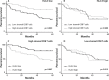
Figure 4. Prognostic benefit in HLA-E negative tumors with high CD8+ T cell infiltration
Patients were divided in two groups with low or high stromal CD8+ T cell infiltration, based on the mean CD8+ T-cell count for all patients and on the expression of HLA-E. Kaplan-Meier curves were constructed to estimate OS of the two groups whereas the log-rank test was used to compare the difference between the two curves. A. The effect of CD8+ T cell infiltration in patients with low HLA-E expression (n=77), showing that a high stromal CD8+ T cell infiltration was strongly associated with a better OS. B. The effect of a high stromal CD8+ T cell infiltrate was neutralized in tumors with high HLA-E expression (n=86). C, D. Conversely, in patients with high stromal CD8+ T cell influx (n=71), a high HLA-E expression is associated with a worse OS (C). In patients with low presence of stromal CD8+ T cells (n=92), HLA-E expression had no effect on OS (D).
Similar articles
- Predominant infiltration of macrophages and CD8(+) T Cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer.
Kawai O, Ishii G, Kubota K, Murata Y, Naito Y, Mizuno T, Aokage K, Saijo N, Nishiwaki Y, Gemma A, Kudoh S, Ochiai A. Kawai O, et al. Cancer. 2008 Sep 15;113(6):1387-95. doi: 10.1002/cncr.23712. Cancer. 2008. PMID: 18671239 - Prognostic Significance of Programmed Cell Death Ligand 1 (PD-L1), CD8+ Tumor-Infiltrating Lymphocytes and p53 in Non-Small Cell Lung Cancer: An Immunohistochemical Study.
Rashed HE, Abdelrahman AE, Abdelgawad M, Balata S, Shabrawy ME. Rashed HE, et al. Turk Patoloji Derg. 2017;1(1):211-222. doi: 10.5146/tjpath.2017.01398. Turk Patoloji Derg. 2017. PMID: 28832075 English. - Stromal CD8+ T-cell Density—A Promising Supplement to TNM Staging in Non-Small Cell Lung Cancer.
Donnem T, Hald SM, Paulsen EE, Richardsen E, Al-Saad S, Kilvaer TK, Brustugun OT, Helland A, Lund-Iversen M, Poehl M, Olsen KE, Ditzel HJ, Hansen O, Al-Shibli K, Kiselev Y, Sandanger TM, Andersen S, Pezzella F, Bremnes RM, Busund LT. Donnem T, et al. Clin Cancer Res. 2015 Jun 1;21(11):2635-43. doi: 10.1158/1078-0432.CCR-14-1905. Epub 2015 Feb 13. Clin Cancer Res. 2015. PMID: 25680376 - Prognostic value of survivin expression in stage III non-small cell lung cancer patients treated with platinum-based therapy.
Cho HJ, Kim HR, Park YS, Kim YH, Kim DK, Park SI. Cho HJ, et al. Surg Oncol. 2015 Dec;24(4):329-34. doi: 10.1016/j.suronc.2015.09.001. Epub 2015 Sep 14. Surg Oncol. 2015. PMID: 26690822 Review. - The transition from HLA-I positive to HLA-I negative primary tumors: the road to escape from T-cell responses.
Aptsiauri N, Ruiz-Cabello F, Garrido F. Aptsiauri N, et al. Curr Opin Immunol. 2018 Apr;51:123-132. doi: 10.1016/j.coi.2018.03.006. Epub 2018 Mar 19. Curr Opin Immunol. 2018. PMID: 29567511 Review.
Cited by
- A cuproptosis score model and prognostic score model can evaluate clinical characteristics and immune microenvironment in NSCLC.
Tang Y, Wang T, Li Q, Shi J. Tang Y, et al. Cancer Cell Int. 2024 Feb 10;24(1):68. doi: 10.1186/s12935-024-03267-8. Cancer Cell Int. 2024. PMID: 38341588 Free PMC article. - HLA-E expression and its clinical relevance in human renal cell carcinoma.
Seliger B, Jasinski-Bergner S, Quandt D, Stoehr C, Bukur J, Wach S, Legal W, Taubert H, Wullich B, Hartmann A. Seliger B, et al. Oncotarget. 2016 Oct 11;7(41):67360-67372. doi: 10.18632/oncotarget.11744. Oncotarget. 2016. PMID: 27589686 Free PMC article. - Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells.
André P, Denis C, Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, Bléry M, Bonnafous C, Gauthier L, Morel A, Rossi B, Remark R, Breso V, Bonnet E, Habif G, Guia S, Lalanne AI, Hoffmann C, Lantz O, Fayette J, Boyer-Chammard A, Zerbib R, Dodion P, Ghadially H, Jure-Kunkel M, Morel Y, Herbst R, Narni-Mancinelli E, Cohen RB, Vivier E. André P, et al. Cell. 2018 Dec 13;175(7):1731-1743.e13. doi: 10.1016/j.cell.2018.10.014. Epub 2018 Nov 29. Cell. 2018. PMID: 30503213 Free PMC article. Review. - In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target.
Manguso RT, Pope HW, Zimmer MD, Brown FD, Yates KB, Miller BC, Collins NB, Bi K, LaFleur MW, Juneja VR, Weiss SA, Lo J, Fisher DE, Miao D, Van Allen E, Root DE, Sharpe AH, Doench JG, Haining WN. Manguso RT, et al. Nature. 2017 Jul 27;547(7664):413-418. doi: 10.1038/nature23270. Epub 2017 Jul 19. Nature. 2017. PMID: 28723893 Free PMC article. - Implications of NKG2A in immunity and immune-mediated diseases.
Wang X, Xiong H, Ning Z. Wang X, et al. Front Immunol. 2022 Aug 10;13:960852. doi: 10.3389/fimmu.2022.960852. eCollection 2022. Front Immunol. 2022. PMID: 36032104 Free PMC article. Review.
References
- Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. - PubMed
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
- van der Drift MA, Karim-Kos HE, Siesling S, Groen HJ, Wouters MW, Coebergh JW, de VE, Janssen-Heijnen ML. Progress in standard of care therapy and modest survival benefits in the treatment of non-small cell lung cancer patients in the Netherlands in the last 20 years. J Thorac Oncol. 2012;7:291–298. - PubMed
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials