Deletion of 5-Lipoxygenase in the Tumor Microenvironment Promotes Lung Cancer Progression and Metastasis through Regulating T Cell Recruitment - PubMed (original) (raw)
Deletion of 5-Lipoxygenase in the Tumor Microenvironment Promotes Lung Cancer Progression and Metastasis through Regulating T Cell Recruitment
Joanna M Poczobutt et al. J Immunol. 2016.
Abstract
Eicosanoids, including PGs, produced by cyclooxygenases (COX), and leukotrienes, produced by 5-lipoxygenase (5-LO) have been implicated in cancer progression. These molecules are produced by both cancer cells and the tumor microenvironment (TME). We previously reported that both COX and 5-LO metabolites increase during progression in an orthotopic immunocompetent model of lung cancer. Although PGs in the TME have been well studied, less is known regarding 5-LO products produced by the TME. We examined the role of 5-LO in the TME using a model in which Lewis lung carcinoma cells are directly implanted into the lungs of syngeneic WT mice or mice globally deficient in 5-LO (5-LO-KO). Unexpectedly, primary tumor volume and liver metastases were increased in 5-LO-KO mice. This was associated with an ablation of leukotriene (LT) production, consistent with production mainly mediated by the microenvironment. Increased tumor progression was partially reproduced in global LTC4 synthase KO or mice transplanted with LTA4 hydrolase-deficient bone marrow. Tumor-bearing lungs of 5-LO-KO had decreased numbers of CD4 and CD8 T cells compared with WT controls, as well as fewer dendritic cells. This was associated with lower levels of CCL20 and CXL9, which have been implicated in dendritic and T cell recruitment. Depletion of CD8 cells increased tumor growth and eliminated the differences between WT and 5-LO mice. These data reveal an antitumorigenic role for 5-LO products in the microenvironment during lung cancer progression through regulation of T cells and suggest that caution should be used in targeting this pathway in lung cancer.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures
FIGURE 1.
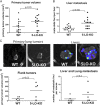
5-LO deficiency in the tumor microenvironment increases primary tumor growth and liver metastasis burden. Murine lung adenocarcinoma cells, LLC-Luc, which possess the WT 5-LO gene and were engineered to express luciferase, were injected directly into the left lung lobe of syngeneic WT or 5-LO-KO mice. Animals were sacrificed 3 wk after injection. Prior to euthanasia, mice were injected with luciferin, and metastases were visualized by bioluminescence in excised organs. Primary tumor was measured using digital caliper. Data represent two separate experiments. Each dot represents a single mouse. Bars indicate mean levels. (A) Primary tumor volumes. (B) Number of metastatic foci per liver. (C) Bioluminescent images of livers with high metastatic burden in WT or 5-LO-KO mice. (D) Flank injection model. LLC-Luc cells were injected in flanks of WT and 5-LO-KO mice and sacrificed 3 wk after injection. Left panel, Primary (flank) tumor volumes measured by caliper. Right panel, Combined number of lung and liver metastatic foci per mouse, measured by bioluminescence after organ harvest.
FIGURE 2.
Expression of 5-LO in the microenvironment is required for the production of leukotrienes in tumor-bearing lungs. WT and 5-LO-KO mice were implanted in the left lung with LLC-Luc cells and sacrificed 3 wk after injection. Eicosanoids were extracted from tumor-bearing left lungs, quantified by LC/MS/MS, and normalized to the protein content of the sample. Bars indicate the mean level. Each dot represents a single mouse. (A) Products of the 5-LO pathway. (B) Products of the COX pathway.
FIGURE 3.
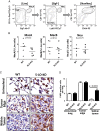
The numbers of myeloid cells in tumor-bearing lungs are not affected by 5-LO deficiency in the TME. (A) Sequential flow cytometry gating strategy to identify myeloid subsets in the lung: MacA macrophages (SigF+/CD11c+), neutrophils (Neu, CD11b+/Ly6G+), and MacB macrophages (SigF−/Ly6G−/CD11b+/CD64+). (B) WT and 5-LO-KO mice were implanted in the left lung with LLC-Luc cells and sacrificed after 2.5 wk. Single-cell suspensions were prepared from tumor-bearing left lobes and the numbers of macrophages (MacA and MacB) and neutrophils analyzed by flow cytometry. Each dot represents a single mouse. Data represent three independent experiments. (C) Macrophage localization in tumor-bearing lungs from WT and 5-LO-KO mice was analyzed by immunohistochemistry using F4/80 as a macrophage marker. Original magnification ×100. (D) Quantification of F4/80-positive cells in uninvolved lung, on the tumor edge, and within the tumor, expressed as the number of positive cells per ×100 field. Sections from four mice per group were counted.
FIGURE 4.
The numbers of lymphocytes in tumor-bearing lungs are decreased in the setting of 5-LO-KO in the TME. (A) Sequential flow cytometry gating strategy to identify lymphocytes in tumor-bearing lungs: NK cells (NK 1.1+/CD3−), CD3 cells (CD3+/SSCLow), CD4 cells (CD3+/CD4+), and CD8 cells (CD3+/CD8+). (B) Flow cytometry–based quantitation of CD3, CD4, and CD8 cells in tumor bearing lungs from WT and 5-LO-KO mice, harvested 2.5 wk after LLC-Luc cells injection. Each dot represents a single mouse. Data represent three separate flow analyses. (C) Quantitation of NK cells and dendritic cells (DC). DC were identified using myeloid marker panel (CD11cHi/MHC class IIHi/SigF−). (D) Number of CD3-positive cells was assessed by immunohistochemistry. Quantification was performed in sections from six WT and four 5-LO-KO mice.
FIGURE 5.
The increase in tumor growth rate in 5-LO-KO mice is dependent on CD8 cells. (A) Decreased expression of lymphocyte-chemoattractant chemokines in tumor-bearing lungs from 5-LO-KO mice. WT and 5-LO-KO mice were implanted in the left lung with LLC-Luc cells and sacrificed 3 wk after injection. Tumor-bearing lungs were snap-frozen and homogenized, and RNA was extracted. Expression levels of CCL20, CXCL9, CXCL10, granzyme B, IFN-γ, MCP-1, and SDF-1 were assessed by quantitative RT-PCR and normalized to 18S. (B) Effects of CD8 cell depletion. WT and 5-LO-KO mice were injected directly in the left lung with LLC-Luc cells and treated with anti-CD8 Ab or isotype control. Tumors were excised 2.5 wk after injection and measured with electronic calipers.
FIGURE 6.
The role of cysteinyl leukotrienes and LTB4 in cancer progression. (A) LLC-Luc cancer cells were injected in the left lung lobe of LTC4S-KO or WT mice. Animals were sacrificed 4 wk after injections. Tumor-bearing lungs were excised and the tumor volume was measured by a caliper. Prior to euthanasia, mice were injected with luciferin to enable visualization of metastatic foci by bioluminescence. Each dot represents a single mouse. Left panel, Tumor volumes; right panel, metastatic foci in the liver. (B) WT mice were irradiated and transplanted with bone marrow (BM) from LTA4H-KO or WT donors. Eight weeks after transplantation, mice were injected with LLC-Luc cancer cells. Three weeks post–cancer cell injection, animals were sacrificed for tumor growth and metastasis assessment (as above). Data represent mice from two separate injections of cancer cells. Left panel, Tumor volumes; right panel, metastatic foci in the liver.
FIGURE 7.
5-LO deficiency in TME leads to increased microvessel formation. Left lung lobes were harvested from WT and 5-LO-KO mice 3 wk after injection of LLC-Luc cancer cells. Microvessel density in histological sections of tumors was assessed using CD31 as a marker of vascular endothelium and quantified as described in Materials and Methods. (A) Representative images of sections from WT and 5-LO-KO mice (original magnification ×40). Brown stain indicates CD31 Ag. (B) Quantification of microvessel density.
Similar articles
- Eicosanoid profiling in an orthotopic model of lung cancer progression by mass spectrometry demonstrates selective production of leukotrienes by inflammatory cells of the microenvironment.
Poczobutt JM, Gijon M, Amin J, Hanson D, Li H, Walker D, Weiser-Evans M, Lu X, Murphy RC, Nemenoff RA. Poczobutt JM, et al. PLoS One. 2013 Nov 11;8(11):e79633. doi: 10.1371/journal.pone.0079633. eCollection 2013. PLoS One. 2013. PMID: 24244531 Free PMC article. - HDAC9 deficiency promotes tumor progression by decreasing the CD8+ dendritic cell infiltration of the tumor microenvironment.
Ning Y, Ding J, Sun X, Xie Y, Su M, Ma C, Pan J, Chen J, Jiang H, Qi C. Ning Y, et al. J Immunother Cancer. 2020 Jun;8(1):e000529. doi: 10.1136/jitc-2020-000529. J Immunother Cancer. 2020. PMID: 32554611 Free PMC article. - Hirsutella sinensis Inhibits Lewis Lung Cancer via Tumor Microenvironment Effector T Cells in Mice.
Fu H, Jin L, Shao X, Li Y, Chen F, Shou Z, Tang X, Ji B, Shou Q. Fu H, et al. Am J Chin Med. 2018;46(4):911-922. doi: 10.1142/S0192415X18500489. Epub 2018 May 13. Am J Chin Med. 2018. PMID: 29754506 - Model for mediastinal lymph node metastasis produced by orthotopic intrapulmonary implantation of lung cancer cells in mice.
Yamaura T, Doki Y, Murakami K, Saiki I. Yamaura T, et al. Hum Cell. 1999 Dec;12(4):197-204. Hum Cell. 1999. PMID: 10834106 Review. - Natural Products with Activity against Lung Cancer: A Review Focusing on the Tumor Microenvironment.
Yang Y, Li N, Wang TM, Di L. Yang Y, et al. Int J Mol Sci. 2021 Oct 7;22(19):10827. doi: 10.3390/ijms221910827. Int J Mol Sci. 2021. PMID: 34639167 Free PMC article. Review.
Cited by
- Neutrophils in the premetastatic niche: key functions and therapeutic directions.
Jia J, Wang Y, Li M, Wang F, Peng Y, Hu J, Li Z, Bian Z, Yang S. Jia J, et al. Mol Cancer. 2024 Sep 14;23(1):200. doi: 10.1186/s12943-024-02107-7. Mol Cancer. 2024. PMID: 39277750 Free PMC article. Review. - Communication between human macrophages and epithelial cancer cell lines dictates lipid mediator biosynthesis.
Werner M, Pace S, Czapka A, Jordan PM, Gerstmeier J, Koeberle A, Werz O. Werner M, et al. Cell Mol Life Sci. 2020 Nov;77(21):4365-4378. doi: 10.1007/s00018-019-03413-w. Epub 2020 Jan 1. Cell Mol Life Sci. 2020. PMID: 31894359 Free PMC article. - Novel EGFR-mutant mouse models of lung adenocarcinoma reveal adaptive immunity requirement for durable osimertinib response.
Kleczko EK, Le AT, Hinz TK, Nguyen TT, Navarro A, Hu CJ, Selman AM, Clambey ET, Merrick DT, Lu S, Weiser-Evans M, Nemenoff RA, Heasley LE. Kleczko EK, et al. Cancer Lett. 2023 Mar 1;556:216062. doi: 10.1016/j.canlet.2023.216062. Epub 2023 Jan 16. Cancer Lett. 2023. PMID: 36657561 Free PMC article. - Complement Activation via a C3a Receptor Pathway Alters CD4+ T Lymphocytes and Mediates Lung Cancer Progression.
Kwak JW, Laskowski J, Li HY, McSharry MV, Sippel TR, Bullock BL, Johnson AM, Poczobutt JM, Neuwelt AJ, Malkoski SP, Weiser-Evans MC, Lambris JD, Clambey ET, Thurman JM, Nemenoff RA. Kwak JW, et al. Cancer Res. 2018 Jan 1;78(1):143-156. doi: 10.1158/0008-5472.CAN-17-0240. Epub 2017 Nov 8. Cancer Res. 2018. PMID: 29118090 Free PMC article. - Activation of PPARγ in Myeloid Cells Promotes Progression of Epithelial Lung Tumors through TGFβ1.
Sippel TR, Johnson AM, Li HY, Hanson D, Nguyen TT, Bullock BL, Poczobutt JM, Kwak JW, Kleczko EK, Weiser-Evans MC, Nemenoff RA. Sippel TR, et al. Mol Cancer Res. 2019 Aug;17(8):1748-1758. doi: 10.1158/1541-7786.MCR-19-0236. Epub 2019 May 14. Mol Cancer Res. 2019. PMID: 31088909 Free PMC article.
References
- Cho W. C., Kwan C. K., Yau S., So P. P., Poon P. C., Au J. S. 2011. The role of inflammation in the pathogenesis of lung cancer. Expert Opin. Ther. Targets 15: 1127–1137. - PubMed
- Peters-Golden M., Henderson W. R., Jr. 2007. Leukotrienes. N. Engl. J. Med. 357: 1841–1854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 CA058187/CA/NCI NIH HHS/United States
- IK2 BX001282/BX/BLRD VA/United States
- T32 HL007171/HL/NHLBI NIH HHS/United States
- R01 CA162226/CA/NCI NIH HHS/United States
- P30 CA046934/CA/NCI NIH HHS/United States
- T32 CA174648/CA/NCI NIH HHS/United States
- R01 CA108610/CA/NCI NIH HHS/United States
- R01 CA164780/CA/NCI NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- T32CA17468/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous