Triple therapy in type 2 diabetes; a systematic review and network meta-analysis - PubMed (original) (raw)
Triple therapy in type 2 diabetes; a systematic review and network meta-analysis
Martin J Downes et al. PeerJ. 2015.
Abstract
Aims. The purpose was to evaluate the evidence for triple therapy regimen using medicines available in Australia for type 2 diabetes. Methods. A systematic literature review was performed to update the relevant evidence from 2002 to 2014 on triple therapy for type 2 diabetes. A multiple-treatments network meta-analysis was undertaken to summarise the comparative efficacy and harms of different triple therapies. Results. Twenty seven trials were identified, most were six months of duration. The following combinations were included in the network meta-analysis: metformin (MET) + sulfonylureas (SU) (used as reference combination); MET + SU+ dipeptidyl peptidase 4 inhibitors (DPP-4-i); MET + SU+ thiazolidinediones (TZD); MET + SU+ glucagon-like peptide-1 receptor agonists (GLP-1-RA); MET + SU+ insulins; MET + TZD + DPP-4-i; and MET + SU+ sodium/glucose cotransporter 2 inhibitors (SGLT2-i). For HbA1c reduction, all triple therapies were statistically superior to MET+SU dual therapy, except for MET + TZD + DPP-4-i. None of the triple therapy combinations demonstrated differences in HbA1c compared with other triple therapies. MET + SU + SGLT2-i and MET + SU + GLP-1-RA resulted in significantly lower body weight than MET + SU + DPP-4-i, MET+SU+insulin and MET + SU + TZDs; MET + SU + DPP-4-i resulted in significantly lower body weight than MET + SU + insulin and MET + SU + TZD. MET + SU + insulin, MET + SU + TZD and MET + SU + DPP-4-i increased the odds of hypoglycaemia when compared to MET + SU. MET + SU + GLP-1-RA reduced the odds of hypoglycaemia compared to MET + SU + insulin. Conclusion. Care when choosing a triple therapy combination is needed as there is often a risk of increased hypoglycaemia events associated with this regimen and there are very limited data surrounding the long-term effectiveness and safety of combined therapies.
Keywords: Anti-diabetic medication; Glycated haemoglobin; Network meta-analysis; Oral antidiabetic drugs; Type 2 diabetes.
Conflict of interest statement
The authors declare there are no competing interests.
Figures
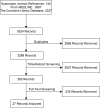
Figure 1. PRISMA flow diagram for RCTs in a systematic review of triple therapy in type 2 diabetes.
Flow diagram showing the total number of records identified and the number of records filtered at each stage of the selection process from the systematic search for randomised control trials of type 2 diabetes in November 2014.
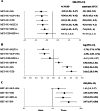
Figure 2. Line plots for different in efficacy and safety outcomes of triple therapy combinations compared to MET + SU dual therapy in type 2 diabetes.
Line (forest) plots of mean difference of change in HbA1c (A), change in body weight (B), and hypoglycaemia (C), for different triple therapy combinations compared to MET + SU dual therapy. Abbreviations: CI, confidence interval; MD, mean difference; DPP-4-i, dipeptidyl peptidase-4 inhibitor; GLP-1-RA, glucagon-like peptide-1 receptor agonist; NGSP, National Glycohemoglobin Standardization Program; IFCC, International Federation of Clinical Chemistry and Laboratory Medicine. HbA1c, glycated haemoglobin; INS, insulin; MET, metformin; PBO, placebo; SU, sulfonylurea; TZD, thiazolidinedione; sodium/glucose cotransporter 2 inhibitors (SGLT2-i).
Similar articles
- Dipeptidyl peptidase-4 inhibitors in triple oral therapy regimens in patients with type 2 diabetes mellitus.
Barnett AH, Charbonnel B, Moses RG, Kalra S. Barnett AH, et al. Curr Med Res Opin. 2015;31(10):1919-31. doi: 10.1185/03007995.2015.1081589. Epub 2015 Sep 11. Curr Med Res Opin. 2015. PMID: 26361231 Review. - Comparative Effectiveness of Adding Alogliptin to Metformin Plus Sulfonylurea with Other DPP-4 Inhibitors in Type 2 Diabetes: A Systematic Review and Network Meta-Analysis.
Kay S, Strickson A, Puelles J, Selby R, Benson E, Tolley K. Kay S, et al. Diabetes Ther. 2017 Apr;8(2):251-273. doi: 10.1007/s13300-017-0245-8. Epub 2017 Mar 8. Diabetes Ther. 2017. PMID: 28275958 Free PMC article. Review. - Glycaemic efficacy of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors as add-on therapy to metformin in subjects with type 2 diabetes-a review and meta analysis.
Deacon CF, Mannucci E, Ahrén B. Deacon CF, et al. Diabetes Obes Metab. 2012 Aug;14(8):762-7. doi: 10.1111/j.1463-1326.2012.01603.x. Epub 2012 Apr 24. Diabetes Obes Metab. 2012. PMID: 22471248 Review. - Efficacy and Safety of DPP-4 Inhibitors and Metformin Combinations in Type 2 Diabetes: A Systematic Literature Review and Network Meta-Analysis.
Chen R, Li J, Chen D, Wen W, Zhang S, Li J, Ruan Y, Zhang Z, Sun J, Chen H. Chen R, et al. Diabetes Metab Syndr Obes. 2024 Jun 19;17:2471-2493. doi: 10.2147/DMSO.S450994. eCollection 2024. Diabetes Metab Syndr Obes. 2024. PMID: 38910912 Free PMC article.
Cited by
- Diabetes mellitus increased integrins gene expression in rat endometrium at the time of embryo implantation.
Bakhteyari Ph D Candidate A, Zarrin Y, Nikpour P, Sadat Hosseiny Z, Sadat Mostafavi F, Eskandari N, Matinfar M, Aboutorabi R. Bakhteyari Ph D Candidate A, et al. Int J Reprod Biomed. 2019 Jul 29;17(6):395-404. doi: 10.18502/ijrm.v17i6.4810. eCollection 2019 Jun. Int J Reprod Biomed. 2019. PMID: 31508564 Free PMC article. - Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD).
Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, Rosas SE, Del Prato S, Mathieu C, Mingrone G, Rossing P, Tankova T, Tsapas A, Buse JB. Davies MJ, et al. Diabetes Care. 2022 Nov 1;45(11):2753-2786. doi: 10.2337/dci22-0034. Diabetes Care. 2022. PMID: 36148880 Free PMC article. Review. - Teneligliptin versus sitagliptin in Korean patients with type 2 diabetes inadequately controlled with metformin and glimepiride: A randomized, double-blind, non-inferiority trial.
Kim Y, Kang ES, Jang HC, Kim DJ, Oh T, Kim ES, Kim NH, Choi KM, Kim SR, You J, Kim SJ, Lee MK. Kim Y, et al. Diabetes Obes Metab. 2019 Mar;21(3):631-639. doi: 10.1111/dom.13566. Epub 2018 Nov 22. Diabetes Obes Metab. 2019. PMID: 30362280 Free PMC article. Clinical Trial. - Combination therapy of oral hypoglycemic agents in patients with type 2 diabetes mellitus.
Moon MK, Hur KY, Ko SH, Park SO, Lee BW, Kim JH, Rhee SY, Kim HJ, Choi KM, Kim NH; Committee of Clinical Practice Guidelines of the Korean Diabetes Association. Moon MK, et al. Korean J Intern Med. 2017 Nov;32(6):974-983. doi: 10.3904/kjim.2017.354. Epub 2017 Oct 27. Korean J Intern Med. 2017. PMID: 29096431 Free PMC article. Review. - Auricular Acupuncture for Lowering Blood Glucose in Type 2 Diabetes: A Pilot Study.
Boccino J. Boccino J. Med Acupunct. 2023 Aug 1;35(4):186-194. doi: 10.1089/acu.2023.0022. Epub 2023 Aug 14. Med Acupunct. 2023. PMID: 37609549 Free PMC article.
References
- Australian Government DoH . Public Summary Document for Vildagliptin, tablet, 50 mg, Galvus®—March 2010. Canberra: Pharmaceutical Evaluation Branch, Department of Health; 2010. Available at http://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/psd/2... (accessed 30 April 2015)
- Australian Government DoH . Canberra: Pharmaceutical Evaluation Branch, Department of Health; 2013. Request for quotation for the provision of a report on the comparative safety and effectiveness of type 2 diabetes medicines. (04/30/2015). RFQ No. 067/1314.
- Bennett WL, Wilson LM, Bolen S, Maruthur N, Singh S, Chatterjee R, Marinopoulos SS, Puhan MA, Ranasinghe P, Nicholson WK, Block L, Odelola O, Dalal DS, Ogbeche GE, Chandrasekhar A, Hutfless S, Bass EB, Segal JB. Oral diabetes medications for adults with type 2 diabetes: an update. Agency for Healthcare Research and Quality, RockvilleComparative effectiveness review No. 27. (Prepared by Johns Hopkins University Evidence-based Practice Center under Contract No. 290-02-0018.) AHRQ Publication No. 11-EHC038-EF. 2011 Available at www.effectivehealthcare.ahrq.gov/reports/final.cfm . - PubMed
- Bergenstal R, Lewin A, Bailey T, Chang D, Gylvin T, Roberts V, NovoLog Mix-vs.-Exenatide Study G Efficacy and safety of biphasic insulin aspart 70/30 versus exenatide in subjects with type 2 diabetes failing to achieve glycemic control with metformin and a sulfonylurea. Current Medical Research and Opinion. 2009;25(1):65–75. doi: 10.1185/03007990802597951. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous