Risks and Benefits of Nalmefene in the Treatment of Adult Alcohol Dependence: A Systematic Literature Review and Meta-Analysis of Published and Unpublished Double-Blind Randomized Controlled Trials - PubMed (original) (raw)
Review
Risks and Benefits of Nalmefene in the Treatment of Adult Alcohol Dependence: A Systematic Literature Review and Meta-Analysis of Published and Unpublished Double-Blind Randomized Controlled Trials
Clément Palpacuer et al. PLoS Med. 2015.
Abstract
Background: Nalmefene is a recent option in alcohol dependence treatment. Its approval was controversial. We conducted a systematic review and meta-analysis of the aggregated data (registered as PROSPERO 2014:CRD42014014853) to compare the harm/benefit of nalmefene versus placebo or active comparator in this indication.
Methods and findings: Three reviewers searched for published and unpublished studies in Medline, the Cochrane Library, Embase, ClinicalTrials.gov, Current Controlled Trials, and bibliographies and by mailing pharmaceutical companies, the European Medicines Agency (EMA), and the US Food and Drug Administration. Double-blind randomized clinical trials evaluating nalmefene to treat adult alcohol dependence, irrespective of the comparator, were included if they reported (1) health outcomes (mortality, accidents/injuries, quality of life, somatic complications), (2) alcohol consumption outcomes, (3) biological outcomes, or (4) treatment safety outcomes, at 6 mo and/or 1 y. Three authors independently screened the titles and abstracts of the trials identified. Relevant trials were evaluated in full text. The reviewers independently assessed the included trials for methodological quality using the Cochrane Collaboration tool for assessing risk of bias. On the basis of the I2 index or the Cochrane's Q test, fixed or random effect models were used to estimate risk ratios (RRs), mean differences (MDs), or standardized mean differences (SMDs) with 95% CIs. In sensitivity analyses, outcomes for participants who were lost to follow-up were included using baseline observation carried forward (BOCF); for binary measures, patients lost to follow-up were considered equal to failures (i.e., non-assessed patients were recorded as not having responded in both groups). Five randomized controlled trials (RCTs) versus placebo, with a total of 2,567 randomized participants, were included in the main analysis. None of these studies was performed in the specific population defined by the EMA approval of nalmefene, i.e., adults with alcohol dependence who consume more than 60 g of alcohol per day (for men) or more than 40 g per day (for women). No RCT compared nalmefene with another medication. Mortality at 6 mo (RR = 0.39, 95% CI [0.08; 2.01]) and 1 y (RR = 0.98, 95% CI [0.04; 23.95]) and quality of life at 6 mo (SF-36 physical component summary score: MD = 0.85, 95% CI [-0.32; 2.01]; SF-36 mental component summary score: MD = 1.01, 95% CI [-1.33; 3.34]) were not different across groups. Other health outcomes were not reported. Differences were encountered for alcohol consumption outcomes such as monthly number of heavy drinking days at 6 mo (MD = -1.65, 95% CI [-2.41; -0.89]) and at 1 y (MD = -1.60, 95% CI [-2.85; -0.35]) and total alcohol consumption at 6 mo (SMD = -0.20, 95% CI [-0.30; -0.10]). An attrition bias could not be excluded, with more withdrawals for nalmefene than for placebo, including more withdrawals for safety reasons at both 6 mo (RR = 3.65, 95% CI [2.02; 6.63]) and 1 y (RR = 7.01, 95% CI [1.72; 28.63]). Sensitivity analyses showed no differences for alcohol consumption outcomes between nalmefene and placebo, but the weight of these results should not be overestimated, as the BOCF approach to managing withdrawals was used.
Conclusions: The value of nalmefene for treatment of alcohol addiction is not established. At best, nalmefene has limited efficacy in reducing alcohol consumption.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi\_disclosure.pdf (available on request from the corresponding author) and declare that (1) No authors have support from any company for the submitted work; (2) PC was a trainee in Servier (pharmacokinetics department) for 6 months in 2013; LB, BR, RJM, BE have no relationships with any company that might have an interest in the submitted work in the previous 3 years; NF has relationships (Travel/accommodations expenses covered/reimbursed) with Servier, BMS, Lundbeck and Janssen who might have an interest in the work submitted in the previous 3 years, he was invited by Lundbeck to present an oral communication at a symposium on the neurobiology of alcohol dependence in 2014, he explicitly asked not to be paid for this presentation (and indeed was not paid); (3) No author’s spouses, partners, or children have any financial relationships that could be relevant to the submitted work; and (4) none of the authors has any non-financial interests that could be relevant to the submitted work.
Figures
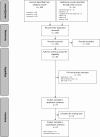
Fig 1. Flow diagram.
FDA, US Food and Drug Administration.
Fig 2. Forest plots for health outcomes at 6 mo.
Comment in
- Meta-analysis finds no evidence for efficacy of nalmefene in treating alcohol dependence.
Mayor S. Mayor S. BMJ. 2015 Dec 29;351:h6988. doi: 10.1136/bmj.h6988. BMJ. 2015. PMID: 26715052 No abstract available.
Similar articles
- Pharmacologically controlled drinking in the treatment of alcohol dependence or alcohol use disorders: a systematic review with direct and network meta-analyses on nalmefene, naltrexone, acamprosate, baclofen and topiramate.
Palpacuer C, Duprez R, Huneau A, Locher C, Boussageon R, Laviolle B, Naudet F. Palpacuer C, et al. Addiction. 2018 Feb;113(2):220-237. doi: 10.1111/add.13974. Epub 2017 Sep 20. Addiction. 2018. PMID: 28940866 - Opioid antagonists for alcohol dependence.
Srisurapanont M, Jarusuraisin N. Srisurapanont M, et al. Cochrane Database Syst Rev. 2005 Jan 25;(1):CD001867. doi: 10.1002/14651858.CD001867.pub2. Cochrane Database Syst Rev. 2005. PMID: 15674887 Updated. Review. - Opioid antagonists for alcohol dependence.
Srisurapanont M, Jarusuraisin N. Srisurapanont M, et al. Cochrane Database Syst Rev. 2002;(2):CD001867. doi: 10.1002/14651858.CD001867. Cochrane Database Syst Rev. 2002. PMID: 12076425 Updated. Review. - Opioid antagonists for alcohol dependence.
Rösner S, Hackl-Herrwerth A, Leucht S, Vecchi S, Srisurapanont M, Soyka M. Rösner S, et al. Cochrane Database Syst Rev. 2010 Dec 8;(12):CD001867. doi: 10.1002/14651858.CD001867.pub3. Cochrane Database Syst Rev. 2010. PMID: 21154349 Review. - Vibration of effects from diverse inclusion/exclusion criteria and analytical choices: 9216 different ways to perform an indirect comparison meta-analysis.
Palpacuer C, Hammas K, Duprez R, Laviolle B, Ioannidis JPA, Naudet F. Palpacuer C, et al. BMC Med. 2019 Sep 16;17(1):174. doi: 10.1186/s12916-019-1409-3. BMC Med. 2019. PMID: 31526369 Free PMC article.
Cited by
- Recommendations of French Alcohol Society and European Federation of Addiction Societies.
Braillon A. Braillon A. CNS Neurosci Ther. 2016 Jun;22(6):535-6. doi: 10.1111/cns.12556. Epub 2016 May 3. CNS Neurosci Ther. 2016. PMID: 27139924 Free PMC article. No abstract available. - Prevention, screening, and treatment for heavy drinking and alcohol use disorder.
Knox J, Hasin DS, Larson FRR, Kranzler HR. Knox J, et al. Lancet Psychiatry. 2019 Dec;6(12):1054-1067. doi: 10.1016/S2215-0366(19)30213-5. Epub 2019 Oct 17. Lancet Psychiatry. 2019. PMID: 31630982 Free PMC article. Review. - Drinking Levels and Profiles of Alcohol Addicted Rats Predict Response to Nalmefene.
Foo JC, Vengeliene V, Noori HR, Yamaguchi I, Morita K, Nakamura T, Yamamoto Y, Spanagel R. Foo JC, et al. Front Pharmacol. 2019 May 7;10:471. doi: 10.3389/fphar.2019.00471. eCollection 2019. Front Pharmacol. 2019. PMID: 31133855 Free PMC article. - The evidence base for psychotropic drugs approved by the European Medicines Agency: a meta-assessment of all European Public Assessment Reports.
Erhel F, Scanff A, Naudet F. Erhel F, et al. Epidemiol Psychiatr Sci. 2020 Apr 27;29:e120. doi: 10.1017/S2045796020000359. Epidemiol Psychiatr Sci. 2020. PMID: 32336312 Free PMC article. - Therapeutic targets in alcohol-associated liver disease: progress and challenges.
Adekunle AD, Adejumo A, Singal AK. Adekunle AD, et al. Therap Adv Gastroenterol. 2023 May 10;16:17562848231170946. doi: 10.1177/17562848231170946. eCollection 2023. Therap Adv Gastroenterol. 2023. PMID: 37187673 Free PMC article. Review.
References
- Marlatt GA, Witkiewitz K. Harm reduction approaches to alcohol use: health promotion, prevention, and treatment. Addict Behav. 2002;27:867–886. - PubMed
- European Medicines Agency. Assessment report: Selincro—international non-proprietory name: nalmefene. EMA/78844/2013. 13 Dec 2012. Available: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_asses.... Accessed 16 November 2015.
- National Institute for Health and Care Excellence. Nalmefene for reducing alcohol consumption in people with alcohol dependence: evaluation report London: National Institute for Health and Care Excellence; 2014.
Publication types
MeSH terms
Substances
Grants and funding
Supported by a local grant from Rennes CHU (CORECT : COmité de la Recherche Clinique et Translationelle). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous