Targeting YAP-Dependent MDSC Infiltration Impairs Tumor Progression - PubMed (original) (raw)
doi: 10.1158/2159-8290.CD-15-0224. Epub 2015 Dec 23.
Xin Lu 1, Prasenjit Dey 1, Pingna Deng 1, Chia Chin Wu 2, Shan Jiang 3, Zhuangna Fang 4, Kun Zhao 3, Ramakrishna Konaparthi 3, Sujun Hua 1, Jianhua Zhang 2, Elsa M Li-Ning-Tapia 5, Avnish Kapoor 3, Chang-Jiun Wu 2, Neelay Bhaskar Patel 3, Zhenglin Guo 6, Vandhana Ramamoorthy 2, Trang N Tieu 2, Tim Heffernan 2, Di Zhao 1, Xiaoying Shang 6, Sunada Khadka 6, Pingping Hou 1, Baoli Hu 1, Eun-Jung Jin 7, Wantong Yao 3, Xiaolu Pan 3, Zhihu Ding 8, Yanxia Shi 4, Liren Li 4, Qing Chang 2, Patricia Troncoso 9, Christopher J Logothetis 5, Mark J McArthur 10, Lynda Chin 3, Y Alan Wang 11, Ronald A DePinho 12
Affiliations
- PMID: 26701088
- PMCID: PMC4707102
- DOI: 10.1158/2159-8290.CD-15-0224
Targeting YAP-Dependent MDSC Infiltration Impairs Tumor Progression
Guocan Wang et al. Cancer Discov. 2016 Jan.
Abstract
The signaling mechanisms between prostate cancer cells and infiltrating immune cells may illuminate novel therapeutic approaches. Here, utilizing a prostate adenocarcinoma model driven by loss of Pten and Smad4, we identify polymorphonuclear myeloid-derived suppressor cells (MDSC) as the major infiltrating immune cell type, and depletion of MDSCs blocks progression. Employing a novel dual reporter prostate cancer model, epithelial and stromal transcriptomic profiling identified CXCL5 as a cancer-secreted chemokine to attract CXCR2-expressing MDSCs, and, correspondingly, pharmacologic inhibition of CXCR2 impeded tumor progression. Integrated analyses identified hyperactivated Hippo-YAP signaling in driving CXCL5 upregulation in cancer cells through the YAP-TEAD complex and promoting MDSC recruitment. Clinicopathologic studies reveal upregulation and activation of YAP1 in a subset of human prostate tumors, and the YAP1 signature is enriched in primary prostate tumor samples with stronger expression of MDSC-relevant genes. Together, YAP-driven MDSC recruitment via heterotypic CXCL5-CXCR2 signaling reveals an effective therapeutic strategy for advanced prostate cancer.
Significance: We demonstrate a critical role of MDSCs in prostate tumor progression and discover a cancer cell nonautonomous function of the Hippo-YAP pathway in regulation of CXCL5, a ligand for CXCR2-expressing MDSCs. Pharmacologic elimination of MDSCs or blocking the heterotypic CXCL5-CXCR2 signaling circuit elicits robust antitumor responses and prolongs survival.
©2015 American Association for Cancer Research.
Conflict of interest statement
Conflict of Interest: The authors disclose no potential conflicts of interest.
Figures
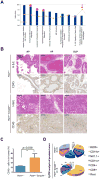
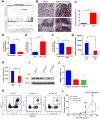
Figure 1. Prominent Infiltration of Immune Cells in the Ptenpc-/-Smad4pc-/- Tumors as Compared to Ptenpc-/- Tumors
(A) The top 10 activated pathways in Ptenpc-/-Smad4pc-/- tumors (n=5) as compared to Ptenpc-/- tumors (n=5) identified by Ingenuity pathway analysis (IPA). (B) A significant increase in the infiltration of immune cells as shown by IHC for CD45 in Ptenpc-/-Smad4pc-/- tumors as compared to Ptenpc-/- tumors from 16 weeks old mice (n=3). AP, VP and DLP stand for anterior prostate, ventral prostate and dorsolateral prostate, respectively. Scale bar 200 μm. (C) Quantification of tumor-infiltrating CD45+ cells (AP, VP and DLP combined) in Ptenpc-/- tumors and Ptenpc-/-Smad4pc-/- from 16 weeks old mice (n=3), assessed by CyTOF. (D) Percentages of various immune cell populations within the CD45+ infiltrating immune cells in prostate tumors from 16 weeks old Ptenpc-/- and Ptenpc-/-Smad4pc-/- mice, assessed with CyTOF (9-marker) and analyzed with Flowjo. CD11b+ myeloid cells are significantly more in Ptenpc-/-Smad4pc-/- tumor as compared to Ptenpc-/- tumor (n=3, P<0.05).
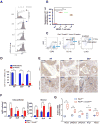
Figure 2
CD11b+ Gr-1+ Cells are Significantly Increased in Ptenpc-/-Smad4pc-/- Tumors as Compared to Ptenpc-/- Tumors. (A) SPADE tree derived from CyTOF (17-marker) analysis of whole tumor cell population from Ptenpc-/-Smad4pc-/- mice at 5-week, 8-week, and 14-week old (n=3). Live single cells were used to construct the tree. Cell populations were identified as PCa cells (EpCAM+ CD45-), non-immune TME cells (EpCAM- CD45-), T cells (CD45+ CD3+ TCRβ+), B cells (CD45+ B220+ CD19+), NK cells (CD45+ NK1.1+), dendritic cells (CD45+ CD11c+), putative MDSCs (CD45+ CD11b+ Gr-1+), and macrophages (CD45+ CD11b+ Gr-1-). On the right panels, the tree is colored by the median intensity of individual markers shown on the top to highlight infiltrating immune cells (EpCAM- CD45+), epithelial PCa cells (EpCAM+ CD45-), total myeloid cells (CD45+ CD11b+), and putative MDSCs (CD45+ CD11b+ Gr-1+). (B-C) CyTOF analysis of tumors (B) or peripheral blood (C) from 5, 8, and 14 weeks old Ptenpc-/-Smad4pc-/- mice revealed an age-dependent increase in the MDSCs infiltration. Prostate from wild type (WT) mice at 16-week old was used as control (n=3 for each genotype). See also Supplementary Figure S1.
Figure 3. MDSCs from Ptenpc-/-Smad4pc-/- Tumors Display Potent Immunosuppressive Activities and are dominated by PMN-MDSCs
(A) CD11b+ Gr-1+ cells from Ptenpc-/-Smad4pc-/- tumors display potent immune-suppressive activity towards T cell activation as demonstrated by CFSE dilution assay in triplicate. (B) Summarized result from (A). (C-D) Flow cytometry analysis shows PMN-MDSCs as the major population in the infiltrated MDSCs in established Ptenpc-/-Smad4pc-/- tumors at AP, DLP and VP (n=5). (E-F) A significant increase in Ly-6G+ cells in Ptenpc-/-Smad4pc-/- tumors as compared to the Ptenpc-/- tumors as shown by IHC for Ly-6G and quantified by location of positively stained cells in the intra-epithelial or stromal compartment of the tumor at AP, DLP and VP (n=3). (G) Quantification of the mRNA expression of subunits of NADPH oxidase (Nox2, p40phox, and p47phox), Arg1 and Nos2 in the Ptenpc-/-Smad4pc-/- tumors and the Ptenpc-/- tumors (n=5). In B, D, F and G, *_P<0.05, **P<0.01, ***P<0.001._Also see Supplementary Figure S2.
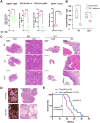
Figure 4. Targeting MDSCs with anti-Gr-1 Neutralizing Antibody or MDSC-specific Peptibody Significantly Delayed Tumor Progression in Ptenpc-/-Smad4pc-/- Mice
(A) Administration of Gr-1 neutralizing antibody in vivo significantly reduced CD45+ infiltrating immune cells, reduced MDSCs and increased CD8+ T cells among total T cells in Ptenpc-/-Smad4pc-/- tumors (n=4), measured by flow cytometry. (B) Gr-1 antibody treatment of 14-week old mice significantly reduced the weight of VP and DLP in Ptenpc-/-Smad4pc-/- mice. (C) Gr-1 antibody remarkably altered the tumor histopathology in Ptenpc-/-Smad4pc-/- adenocarcinoma, analyzed by H&E staining of AP, VP and DLP. (D) One month of Pep-H6 peptibody treatment led to significant appearance and histology changes of the Ptenpc-/-Smad4pc-/- adenocarcinoma. (E) Kaplan-Meier survival curve showing the significant delay of mortality caused by Pep-H6 peptibody treatment of Ptenpc-/-Smad4pc-/- mice. In A and B, *_P<0.05, ***P<0.001._Also see Supplementary Figure S3 and Figure S4.
Figure 5. Cxcl5-Cxcr2 Axis Plays an Indispensable Role in Recruitment of MDSCs and Promotion of Tumor Progression
(A) Establishment of Ptenpc-/-Smad4pc-/-mTmG+ model allows fluorescent visualization of the GFP+ tumor cells intermixed with Tomato+ stroma (Left Panel); FACS isolation of GFP+ tumor cells and Tomato+ stromal cells from the prostate adenocarcinoma (Middle Panel); microarray analysis to identify differentially expressed genes (Right Panel). In the fluorescence image, Bl denotes bladder (completely Tomato+) (n=2). (B) Quantification of mRNA expression shows that Cxcl5 and Cxcr2 were both expressed at higher levels in Ptenpc-/-Smad4pc-/- tumors than Ptenpc-/- tumors, and Cxcl5 expression was enriched in GFP+ tumor cells, whereas Cxcr2 in Tomato+ stromal cells. (n=5) (C) IHC for Cxcl5 showed significantly higher expression levels of Cxcl5 in Ptenpc-/-Smad4pc-/- tumors than Ptenpc-/- tumors (n=3). (D) Blocking the Cxcl5-Cxcr2 axis by Cxcl5 neutralizing antibody, Cxcr2 inhibitor SB225002 or Cxcr2 neutralizing antibody significantly decreased migration of MDSCs towards conditioned medium from Ptenpc-/-Smad4pc-/- tumors cells, evaluated with an in vitro transwell migration assay in triplicate. (E-F) Cxcr2 inhibitor SB225002 treatment of Ptenpc-/-Smad4pc-/- mice for 14 days (n=4) resulted in significantly reduced tumor weight of VP and DLP, and significantly delayed progression for AP prostate cancer shown by H&E staining. (G) Cxcr2 inhibitor SB225002 treatment of Ptenpc-/-Smad4pc-/- mice significantly prolonged their overall survival. In B, D and E, *_P<0.05, **P<0.01, ***P<0.001._Also see Supplementary Figure S5.
Figure 6. Hyperactivation of Yap1 in Ptenpc-/-Smad4pc-/- Tumors Upregulates Cxcl5
(A) GSEA analysis identified Yap1 oncogenic signature as the top activated pathways in the Ptenpc-/-Smad4pc-/- tumors compared to Ptenpc-/- tumors (n=5). (B) A significant increase in nuclear staining of Yap1 in the Ptenpc-/-Smad4pc-/- tumors compared to Ptenpc-/- tumors (n=3). (C) Chromatin immunoprecipitation shows that Yap1 can directly bind to Cxcl5 promoter using quantitative PCR in triplicates. (D) shRNA knockdown of Yap1 in Ptenpc-/-Smad4pc-/- tumor cells resulted in a dramatic reduction in Cxcl5 mRNA expression using quantitative PCR in triplicates. (E) Overexpression of a constitutively active YAP1 S127A mutant resulted in upregulation of Cxcl5 mRNA using quantitative PCR in triplicates. (F) TEAD-binding defective S127A/S94A Yap1 mutant significantly decreased Cxcl5 mRNA expression as compared to the Yap1 S127A mutant using quantitative PCR in triplicates. (G-H) Conditioned medium prepared from _Ptenpc-/-Smad4pc-/-_cells infected with Yap1 shRNA (G) or treated with Verteporfin (H), a small molecule that disrupts Yap1-TEAD interaction, induced less MDSCs migration in vitro as compared to the control conditioned medium. Transwell migration was done in triplicate for each condition. (I) Western blot analysis showed that two independent inducible shRNAs for Yap1 efficiently knockdown Yap1 expression in the _Ptenpc-/-Smad4pc-/-_cells. (J-L) Inducible Yap1 knockdown strongly suppressed the intratumoral MDSC infiltration (J-K) and tumor growth (L) of the C57BL/6-syngeneic cell line isolated from prostate tumor of Ptenpc-/-Smad4pc-/-Trp53pc-/- mice (n=5). In C, D, E and F, G, H, L **P<0.01, ***P<0.001. See also Supplementary Figure S6.
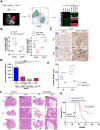
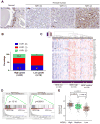
Figure 7. YAP1 is activated in human prostate cancer and correlated with MDSCs signature and CXCL6 overexpression
(A) IHC analysis of YAP1 expression in basal cells of normal prostate tissue and human prostate cancers. Numbers in parenthesis indicates YAP1 IHC intensity scores. (B) YAP1 IHC intensity score representation in low grade (n=10) and high grade (n=60) prostate cancer. (C) Clustering of human TCGA prostate samples into MDSC-high, MDSC-low and MDSC-medium groups using a 39-gene MDSC signature. (D) YAP1 signatures are identified in MDSC-high prostate TCGA samples. (E) CXCL6 expression is significantly higher in MDSC-high group. See also Supplementary Figure S7.
Similar articles
- Myeloid-Derived Suppressor Cell Subset Accumulation in Renal Cell Carcinoma Parenchyma Is Associated with Intratumoral Expression of IL1β, IL8, CXCL5, and Mip-1α.
Najjar YG, Rayman P, Jia X, Pavicic PG Jr, Rini BI, Tannenbaum C, Ko J, Haywood S, Cohen P, Hamilton T, Diaz-Montero CM, Finke J. Najjar YG, et al. Clin Cancer Res. 2017 May 1;23(9):2346-2355. doi: 10.1158/1078-0432.CCR-15-1823. Epub 2016 Oct 31. Clin Cancer Res. 2017. PMID: 27799249 Free PMC article. - CXCR2/CXCL5 axis contributes to epithelial-mesenchymal transition of HCC cells through activating PI3K/Akt/GSK-3β/Snail signaling.
Zhou SL, Zhou ZJ, Hu ZQ, Li X, Huang XW, Wang Z, Fan J, Dai Z, Zhou J. Zhou SL, et al. Cancer Lett. 2015 Mar 28;358(2):124-135. doi: 10.1016/j.canlet.2014.11.044. Epub 2014 Nov 24. Cancer Lett. 2015. PMID: 25462858 - CXCL2/MIF-CXCR2 signaling promotes the recruitment of myeloid-derived suppressor cells and is correlated with prognosis in bladder cancer.
Zhang H, Ye YL, Li MX, Ye SB, Huang WR, Cai TT, He J, Peng JY, Duan TH, Cui J, Zhang XS, Zhou FJ, Wang RF, Li J. Zhang H, et al. Oncogene. 2017 Apr;36(15):2095-2104. doi: 10.1038/onc.2016.367. Epub 2016 Oct 10. Oncogene. 2017. PMID: 27721403 - CXCL5/CXCR2 axis in tumor microenvironment as potential diagnostic biomarker and therapeutic target.
Zhang W, Wang H, Sun M, Deng X, Wu X, Ma Y, Li M, Shuoa SM, You Q, Miao L. Zhang W, et al. Cancer Commun (Lond). 2020 Mar;40(2-3):69-80. doi: 10.1002/cac2.12010. Cancer Commun (Lond). 2020. PMID: 32237072 Free PMC article. Review. - The Hippo Signaling Pathway in Pancreatic Cancer.
Ansari D, Ohlsson H, Althini C, Bauden M, Zhou Q, Hu D, Andersson R. Ansari D, et al. Anticancer Res. 2019 Jul;39(7):3317-3321. doi: 10.21873/anticanres.13474. Anticancer Res. 2019. PMID: 31262852 Review.
Cited by
- Yes-associated protein mediates immune reprogramming in pancreatic ductal adenocarcinoma.
Murakami S, Shahbazian D, Surana R, Zhang W, Chen H, Graham GT, White SM, Weiner LM, Yi C. Murakami S, et al. Oncogene. 2017 Mar 2;36(9):1232-1244. doi: 10.1038/onc.2016.288. Epub 2016 Aug 22. Oncogene. 2017. PMID: 27546622 Free PMC article. - Targeting the secreted RGDKGE collagen fragment reduces PD‑L1 by a proteasome‑dependent mechanism and inhibits tumor growth.
Caron JM, Han X, Lary CW, Sathyanarayana P, Remick SC, Ernstoff MS, Herlyn M, Brooks PC. Caron JM, et al. Oncol Rep. 2023 Feb;49(2):44. doi: 10.3892/or.2023.8481. Epub 2023 Jan 12. Oncol Rep. 2023. PMID: 36633146 Free PMC article. - The potential role of YAP in Axl-mediated resistance to EGFR tyrosine kinase inhibitors.
Saab S, Chang OS, Nagaoka K, Hung MC, Yamaguchi H. Saab S, et al. Am J Cancer Res. 2019 Dec 1;9(12):2719-2729. eCollection 2019. Am J Cancer Res. 2019. PMID: 31911857 Free PMC article. - Hypoxia-mediated stabilization of HIF1A in prostatic intraepithelial neoplasia promotes cell plasticity and malignant progression.
Abu El Maaty MA, Terzic J, Keime C, Rovito D, Lutzing R, Yanushko D, Parisotto M, Grelet E, Namer IJ, Lindner V, Laverny G, Metzger D. Abu El Maaty MA, et al. Sci Adv. 2022 Jul 22;8(29):eabo2295. doi: 10.1126/sciadv.abo2295. Epub 2022 Jul 22. Sci Adv. 2022. PMID: 35867798 Free PMC article. - The emerging roles of Gα12/13 proteins on the hallmarks of cancer in solid tumors.
Rasheed SAK, Subramanyan LV, Lim WK, Udayappan UK, Wang M, Casey PJ. Rasheed SAK, et al. Oncogene. 2022 Jan;41(2):147-158. doi: 10.1038/s41388-021-02069-w. Epub 2021 Oct 23. Oncogene. 2022. PMID: 34689178 Free PMC article. Review.
References
- Karlou M, Tzelepi V, Efstathiou E. Therapeutic targeting of the prostate cancer microenvironment. Nat Rev Urol. 2010;7:494–509. - PubMed
- Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–54. - PubMed
- Hanahan D, Coussens L. Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell. 2012;21:309–22. - PubMed
- Hanahan D, Weinberg Robert A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016672/CA/NCI NIH HHS/United States
- R37 CA084628/CA/NCI NIH HHS/United States
- K99 CA194289/CA/NCI NIH HHS/United States
- U01 CA141508/CA/NCI NIH HHS/United States
- U01CA141508/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous