The redundancy of the mammalian heterochromatic compartment - PubMed (original) (raw)
Review
The redundancy of the mammalian heterochromatic compartment
Joan C Ritland Politz et al. Curr Opin Genet Dev. 2016 Apr.
Abstract
Two chromatin compartments are present in most mammalian cells; the first contains primarily euchromatic, early replicating chromatin and the second, primarily late-replicating heterochromatin, which is the subject of this review. Heterochromatin is concentrated in three intranuclear regions: the nuclear periphery, the perinucleolar space and in pericentromeric bodies. We review recent evidence demonstrating that the heterochromatic compartment is critically involved in global nuclear organization and the maintenance of genome stability, and discuss models regarding how this compartment is formed and maintained. We also evaluate our understanding of how heterochromatic sequences (herein named heterochromatic associated regions (HADs)) might be tethered within these regions and review experiments that reveal the stochastic nature of individual HAD positioning within the compartment. These investigations suggest a substantial level of functional redundancy within the heterochromatic compartment.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
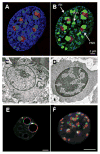
Figure 1. A–B. Heterochromatin distribution in mammalian cell
Murine embryonic fibroblast stained with A) DAPI (blue) plus antibodies to fibrillarin (red) to mark nucleoli and B) antibodies to H3K9me3 (green) and fibrillarin (red). PH = peripheral heterochromatin. PNH = perinucleolar heterochromatin. PCH = pericentromeric heterochromatin. From [2]. C–D. Heterochromatin changes during erythropoiesis. Electron micrographs of C) murine proerythroblast and D) late erythroblast showing change in heterochromatin distribution (arrows) during differentiation. From [6] with permission from Nature Publishing Group. E–F. Heterochromatin changes during early development. E) Single confocal section of mouse preimplantation embryo at early 2 cell and F) 16 cell stage showing distribution of pericentromeric (red) and centromeric chromatin (green). DNA is grey, bars = 5 μm. From [9].
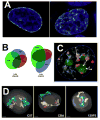
Figure 2. The redundancy of the heterochromatic compartment
A) Redistribution of mother LADs (left, green) in daughter cell (left, green). DAPI staining of chromatin is blue. The unstained areas in the nucleus on the left are nucleoli. Adapted from the graphical abstract in [65], with permission from Elsevier. B) Venn diagrams depict the overlapping association of two late and one early replicating region with the PH, PCH and PNH in human lymphoblastoid nucleus. The size and overlap of the ellipses is proportional to the percent association of the region with each compartment as determined by DNA FISH. From [68]. C) Cartoon overlaid on DAPI-stained nucleus to represent redundant association sites of late replicating DNA regions (yellow dots) in the PH (blue nuclear outline), PCH (red) and PNH (green). Regions can relocalize from PNH to PH after mitosis or upon the loss of nucleoli (arrow with check mark). Shuttling between the PCH and PH or the PNH and PCH has not been studied directly (bidirectional arrows with question marks). From [68]. D) 3D reconstruction and rendering of chromosome paint images from primary B cells from three different mouse strains showing how the presence or absence of an NOR affects chromosome 12 (cyan) and chromosome 15 (yellow) territory position. When NORs are on both chromosomes (C57), territories tend to associate with the nucleolus (orange), if no NOR is present, the territory associates more often with the periphery (CBA: no NOR on chromosome 12; 129P3: no NOR on chromosome 15). DAPI stain is shown in gray. Scale bar = 1 μm. From [72].
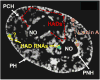
Figure 3. Model depicting redundant distribution of HADs within heterochromatic compartment
Red dashed arrows indicate that most heterochromatic associated domains (HADs) can be found in any of the three heterochromatic regions in different cells or after mitosis. PH: peripheral heterochromatin. PNH: perinucleolar heterochromatin. PCH: pericentromeric heterochromatin. RNAs: RNA adaptors (yellow) transcribed from HADs. PH RNA adaptors have not yet been identified (yellow “?”). Differently colored balls: tethering proteins, some of which are unique to a region (blue and green) and may tether some HADs specifically, and some of which are present in multiple regions (Lamin A, orange). White regions: DAPI staining. Underlying image is midplane of HeLa nucleus acquired using structured illumination microscopy from [74]. Reproduced with permission from Springer. PCH is not obvious in DAPI-stained HeLa nuclei and is labeled here only for illustrative purposes.
Similar articles
- Heterochromatin drives compartmentalization of inverted and conventional nuclei.
Falk M, Feodorova Y, Naumova N, Imakaev M, Lajoie BR, Leonhardt H, Joffe B, Dekker J, Fudenberg G, Solovei I, Mirny LA. Falk M, et al. Nature. 2019 Jun;570(7761):395-399. doi: 10.1038/s41586-019-1275-3. Epub 2019 Jun 5. Nature. 2019. PMID: 31168090 Free PMC article. - Dynamics and anchoring of heterochromatic loci during development.
Thakar R, Gordon G, Csink AK. Thakar R, et al. J Cell Sci. 2006 Oct 15;119(Pt 20):4165-75. doi: 10.1242/jcs.03183. Epub 2006 Sep 19. J Cell Sci. 2006. PMID: 16984972 - Epigenetics of eu- and heterochromatin in inverted and conventional nuclei from mouse retina.
Eberhart A, Feodorova Y, Song C, Wanner G, Kiseleva E, Furukawa T, Kimura H, Schotta G, Leonhardt H, Joffe B, Solovei I. Eberhart A, et al. Chromosome Res. 2013 Aug;21(5):535-54. doi: 10.1007/s10577-013-9375-7. Epub 2013 Aug 31. Chromosome Res. 2013. PMID: 23996328 - Heterochromatin instability in cancer: from the Barr body to satellites and the nuclear periphery.
Carone DM, Lawrence JB. Carone DM, et al. Semin Cancer Biol. 2013 Apr;23(2):99-108. doi: 10.1016/j.semcancer.2012.06.008. Epub 2012 Jun 18. Semin Cancer Biol. 2013. PMID: 22722067 Free PMC article. Review. - Euchromatic and heterochromatic domains at Drosophila telomeres.
Biessmann H, Prasad S, Walter MF, Mason JM. Biessmann H, et al. Biochem Cell Biol. 2005 Aug;83(4):477-85. doi: 10.1139/o05-053. Biochem Cell Biol. 2005. PMID: 16094451 Review.
Cited by
- The p150N domain of chromatin assembly factor-1 regulates Ki-67 accumulation on the mitotic perichromosomal layer.
Matheson TD, Kaufman PD. Matheson TD, et al. Mol Biol Cell. 2017 Jan 1;28(1):21-29. doi: 10.1091/mbc.E16-09-0659. Epub 2016 Nov 2. Mol Biol Cell. 2017. PMID: 27807046 Free PMC article. - Loss of H3K9 trimethylation alters chromosome compaction and transcription factor retention during mitosis.
Djeghloul D, Dimond A, Cheriyamkunnel S, Kramer H, Patel B, Brown K, Montoya A, Whilding C, Wang YF, Futschik ME, Veland N, Montavon T, Jenuwein T, Merkenschlager M, Fisher AG. Djeghloul D, et al. Nat Struct Mol Biol. 2023 Apr;30(4):489-501. doi: 10.1038/s41594-023-00943-7. Epub 2023 Mar 20. Nat Struct Mol Biol. 2023. PMID: 36941433 Free PMC article. - The Sound of Silence: How Silenced Chromatin Orchestrates the Repair of Double-Strand Breaks.
Kendek A, Wensveen MR, Janssen A. Kendek A, et al. Genes (Basel). 2021 Sep 15;12(9):1415. doi: 10.3390/genes12091415. Genes (Basel). 2021. PMID: 34573397 Free PMC article. Review. - Nuclear envelope dysfunction and its contribution to the aging process.
Martins F, Sousa J, Pereira CD, da Cruz E Silva OAB, Rebelo S. Martins F, et al. Aging Cell. 2020 May;19(5):e13143. doi: 10.1111/acel.13143. Epub 2020 Apr 15. Aging Cell. 2020. PMID: 32291910 Free PMC article. Review. - Nucleolus and centromere Tyramide Signal Amplification-Seq reveals variable localization of heterochromatin in different cell types.
Kumar P, Gholamalamdari O, Zhang Y, Zhang L, Vertii A, van Schaik T, Peric-Hupkes D, Sasaki T, Gilbert DM, van Steensel B, Ma J, Kaufman PD, Belmont AS. Kumar P, et al. Commun Biol. 2024 Sep 13;7(1):1135. doi: 10.1038/s42003-024-06838-7. Commun Biol. 2024. PMID: 39271748 Free PMC article.
References
- Beisel C, Paro R. Silencing chromatin: comparing modes and mechanisms. Nat Rev Genet. 2011;12:123–135. - PubMed
- Lemaitre C, Bickmore WA. Chromatin at the nuclear periphery and the regulation of genome functions. Histochem Cell Biol. 2015;144:111–122. - PubMed
- Padeken J, Heun P. Nucleolus and nuclear periphery: velcro for heterochromatin. Curr Opin Cell Biol. 2014;28:54–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA015704/CA/NCI NIH HHS/United States
- R01 HL065440/HL/NHLBI NIH HHS/United States
- R37 DK044746/DK/NIDDK NIH HHS/United States
- U01 DA040583/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources