The role of microglia and macrophages in glioma maintenance and progression - PubMed (original) (raw)
Review
The role of microglia and macrophages in glioma maintenance and progression
Dolores Hambardzumyan et al. Nat Neurosci. 2016 Jan.
Abstract
There is a growing recognition that gliomas are complex tumors composed of neoplastic and non-neoplastic cells, which each individually contribute to cancer formation, progression and response to treatment. The majority of the non-neoplastic cells are tumor-associated macrophages (TAMs), either of peripheral origin or representing brain-intrinsic microglia, that create a supportive stroma for neoplastic cell expansion and invasion. TAMs are recruited to the glioma environment, have immune functions, and can release a wide array of growth factors and cytokines in response to those factors produced by cancer cells. In this manner, TAMs facilitate tumor proliferation, survival and migration. Through such iterative interactions, a unique tumor ecosystem is established, which offers new opportunities for therapeutic targeting.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Figures
Figure 1
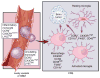
Microglia and monocytes have distinct cellular origins. Under steady-state conditions, these different mononuclear cell populations reside in separate locations. In adult life, monocytes are generated from HSCs that differentiate into granulocyte-macrophage progenitors (GMPs) and then into monocyte-dendritic cell progenitors (MDPs). Mature Ly6Chi CCR2+ CX3CR1low/int inflammatory monocytes are released into circulation, where they can migrate to tissues in response to specific pathological conditions. These cells can also give a rise to circulating monocytes. Microglia originate from yolk sac progenitors in the neuroepithelium beginning around E8.5 in the mouse. In the adult brain, they express high levels of CX3CR1, CD11b and F4/80, but low levels of CD45 and no CCR2. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2015. All rights reserved.
Figure 2
Microglia and monocytes converge in high-grade glioma (HGG). HGG cells induce local inflammation that compromises the integrity of the blood-brain barrier (BBB) and results in Ly6Chi CCR2+ CX3CR1low/int monocytes infiltrating into the tumor. Once in the CNS, these cells can differentiate into tumor-associated macrophages and become nearly indistinguishable from activated resident microglia. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2015. All rights reserved.
Figure 3
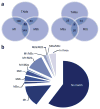
M1/M2 profile of TMAs. Comparison of TAMs with M1- and M2a-, M2b- and M2c-stimulated macrophage data sets (
http://www.ebi.ac.uk/arrayexpress/experiments/E-GEOD-32690/
) containing macrophages stimulated for 24 h in vitro into different polarization states (M0 (unstimulated), M1 (IFNγ + LPS), M2a (IL4), M2b (IFNγ + complexed Ig) and M2c (dexamethasone)), which were compared with TAMs. (a) A graphical representation of the overlap of upregulated genes in TAMs and the four macrophage data sets. The TAMs gene expression profile shows the greatest overlap with M1- and M2b-polarized macrophages. The number of overlapping genes is indicated. (b) Using Gene Set Enrichment Analysis reveals that only a minority of genes that were upregulated in TAMs were also induced in the M1 to M2c phenotype; 59.5% of the genes upregulated in TAMs were not regulated in any of the four macrophage phenotypes.
Figure 4
Glioma cells release several factors, which attract TAMs to the tumor tissue. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2015. All rights reserved.
Figure 5
TAM glioma crosstalk. (a) TAMs release several factors that promote glioma cell invasion. (b) Microglia release TGF-β, which triggers the release of pro-MMP2 from glioma cells. Pro-MMP2 is then cleaved into active MMP2 by microglia-expressed MT1-MMP. Microglial MT1-MMP expression is stimulated by versican, which is released from glioma cells. Versican activates TLR2 and p38- MAP-kinase signaling in microglial cells, which leads to MT1-MMP upregulation. TLR2 signaling in microglia also triggers MMP9 release. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2015. All rights reserved.
Figure 6
Illustration of the complexity and cellular composition of glioma. Gliomas consist of neoplastic tumor cells and non-neoplastic cells from microenvironment, including endothelial cells, pericytes, infiltrating monocytes, activated astrocytes and TAMs. TAMs are recruited to the tumor by tumor bulk and GSCs. These recruited and reprogrammed TAMs secrete soluble factors that both expand the tumor bulk and GSCs as well. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2015. All rights reserved.
Similar articles
- Characterization of microglia/macrophages in gliomas developed in S-100β-v-erbB transgenic rats.
Sasaki A, Yokoo H, Tanaka Y, Homma T, Nakazato Y, Ohgaki H. Sasaki A, et al. Neuropathology. 2013 Oct;33(5):505-14. doi: 10.1111/neup.12015. Epub 2013 Jan 20. Neuropathology. 2013. PMID: 23331472 - Activation of CECR1 in M2-like TAMs promotes paracrine stimulation-mediated glial tumor progression.
Zhu C, Mustafa D, Zheng PP, van der Weiden M, Sacchetti A, Brandt M, Chrifi I, Tempel D, Leenen PJM, Duncker DJ, Cheng C, Kros JM. Zhu C, et al. Neuro Oncol. 2017 May 1;19(5):648-659. doi: 10.1093/neuonc/now251. Neuro Oncol. 2017. PMID: 28453746 Free PMC article. - When Immune Cells Turn Bad-Tumor-Associated Microglia/Macrophages in Glioma.
Roesch S, Rapp C, Dettling S, Herold-Mende C. Roesch S, et al. Int J Mol Sci. 2018 Feb 1;19(2):436. doi: 10.3390/ijms19020436. Int J Mol Sci. 2018. PMID: 29389898 Free PMC article. Review. - Distinct roles of CSF family cytokines in macrophage infiltration and activation in glioma progression and injury response.
Sielska M, Przanowski P, Wylot B, Gabrusiewicz K, Maleszewska M, Kijewska M, Zawadzka M, Kucharska J, Vinnakota K, Kettenmann H, Kotulska K, Grajkowska W, Kaminska B. Sielska M, et al. J Pathol. 2013 Jul;230(3):310-21. doi: 10.1002/path.4192. J Pathol. 2013. PMID: 23520016 - Microglia and central nervous system immunity.
Kaur G, Han SJ, Yang I, Crane C. Kaur G, et al. Neurosurg Clin N Am. 2010 Jan;21(1):43-51. doi: 10.1016/j.nec.2009.08.009. Neurosurg Clin N Am. 2010. PMID: 19944965 Review.
Cited by
- Targeting Tumor-Associated Macrophages in Anti-Cancer Therapies: Convincing the Traitors to Do the Right Thing.
Belgiovine C, Digifico E, Anfray C, Ummarino A, Torres Andón F. Belgiovine C, et al. J Clin Med. 2020 Oct 8;9(10):3226. doi: 10.3390/jcm9103226. J Clin Med. 2020. PMID: 33050070 Free PMC article. Review. - Molecular Heterogeneity and Immunosuppressive Microenvironment in Glioblastoma.
DeCordova S, Shastri A, Tsolaki AG, Yasmin H, Klein L, Singh SK, Kishore U. DeCordova S, et al. Front Immunol. 2020 Jul 17;11:1402. doi: 10.3389/fimmu.2020.01402. eCollection 2020. Front Immunol. 2020. PMID: 32765498 Free PMC article. Review. - Origin, activation, and targeted therapy of glioma-associated macrophages.
Xu C, Xiao M, Li X, Xin L, Song J, Zhan Q, Wang C, Zhang Q, Yuan X, Tan Y, Fang C. Xu C, et al. Front Immunol. 2022 Oct 6;13:974996. doi: 10.3389/fimmu.2022.974996. eCollection 2022. Front Immunol. 2022. PMID: 36275720 Free PMC article. Review. - Phagocytosis Checkpoints in Glioblastoma: CD47 and Beyond.
Afzal A, Afzal Z, Bizink S, Davis A, Makahleh S, Mohamed Y, Coniglio SJ. Afzal A, et al. Curr Issues Mol Biol. 2024 Jul 23;46(8):7795-7811. doi: 10.3390/cimb46080462. Curr Issues Mol Biol. 2024. PMID: 39194679 Free PMC article. Review. - Microglia in Health and Disease: The Strength to Be Diverse and Reactive.
Uriarte Huarte O, Richart L, Mittelbronn M, Michelucci A. Uriarte Huarte O, et al. Front Cell Neurosci. 2021 Mar 31;15:660523. doi: 10.3389/fncel.2021.660523. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33867943 Free PMC article. Review.
References
- Morantz RA, Wood GW, Foster M, Clark M, Gollahon K. Macrophages in experimental and human brain tumors. Part 2: studies of the macrophage content of human brain tumors. J Neurosurg. 1979;50:305–311. - PubMed
- Rossi ML, Hughes JT, Esiri MM, Coakham HB, Brownell DB. Immunohistological study of mononuclear cell infiltrate in malignant gliomas. Acta Neuropathol. 1987;74:269–277. - PubMed
- Hortega PDR. El tercer elemento de los centros nerviosos. Bol Soc Esp d Biol. 1919;9:69–120.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources