Size-dependent control of colloid transport via solute gradients in dead-end channels - PubMed (original) (raw)
Size-dependent control of colloid transport via solute gradients in dead-end channels
Sangwoo Shin et al. Proc Natl Acad Sci U S A. 2016.
Abstract
Transport of colloids in dead-end channels is involved in widespread applications including drug delivery and underground oil and gas recovery. In such geometries, Brownian motion may be considered as the sole mechanism that enables transport of colloidal particles into or out of the channels, but it is, unfortunately, an extremely inefficient transport mechanism for microscale particles. Here, we explore the possibility of diffusiophoresis as a means to control the colloid transport in dead-end channels by introducing a solute gradient. We demonstrate that the transport of colloidal particles into the dead-end channels can be either enhanced or completely prevented via diffusiophoresis. In addition, we show that size-dependent diffusiophoretic transport of particles can be achieved by considering a finite Debye layer thickness effect, which is commonly ignored. A combination of diffusiophoresis and Brownian motion leads to a strong size-dependent focusing effect such that the larger particles tend to concentrate more and reside deeper in the channel. Our findings have implications for all manners of controlled release processes, especially for site-specific delivery systems where localized targeting of particles with minimal dispersion to the nontarget area is essential.
Keywords: colloid; dead-end channel; diffusiophoresis; size effect; solute gradient.
Conflict of interest statement
Conflict of interest statement: P.B.W. discloses a substantive (>$10,000) stock holding in Unilever PLC.
Figures
Fig. 1.
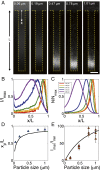
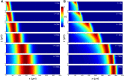
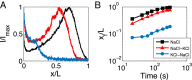
Colloid transport in a dead-end channel induced by a solute gradient. (A) Setup for enabling transport experiments in a dead-end channel with minimum disturbance from a continuous flow. (B) Steplike initial solute and colloid concentrations were realized by inserting an oil droplet or an air bubble in between two liquids with different solute concentrations. (C) Otherwise, a gradual concentration was observed due to mixing. Insets are the fluorescent intensity distributions of colloidal particles (polystyrene latex beads, diameter 0.19 μm) along the dead-end channel. (D) Sequential images and (E) intensity distributions of colloids (particle diameter 0.19 μm) migrating along a dead-end channel in the presence of a solute gradient (NaCl: ci = 2 mM, co = 0.02 mM). Channelwise direction, x, is normalized by the length of the dead-end channel, L (=400 μm). (F) Time taken for the colloidal particles to reach the middle of the dead-end channel with 50% of the inlet fluorescent intensity (0.5Ii) under different solute gradients. (Scale bars: 50 μm.)
Fig. S1.
Measurement of the particle (diameter 1.01 μm) speed along the dead-end channel driven by a solute gradient (NaCl: ci=2 mM, co=0.02 mM). Solid curves represent theoretical results.
Fig. S2.
Schematic illustration of a dead-end channel with length L and height 2h.
Fig. 2.
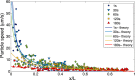
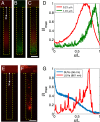
Size-dependent particle focusing driven by a solute gradient (NaCl: ci = 2 mM, co = 0.02 mM). (A) Fluorescent images and (B) intensity distributions of colloids with different diameters ranging from 0.06 to 1.01 μm at t = 300 s. The intensity I is normalized by the maximum intensity Imax. (C) Theoretical prediction for colloid density profiles with different diameters at t = 300 s. Plot of (D) peak position (xp) and (E) focus magnitude (Imax/Imin, Imin is the minimum intensity near the inlet) for various particles at t = 300 s obtained from B. Black curves represent theoretical predictions. (Scale bar: A, 50 μm.)
Fig. S3.
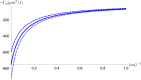
Dependence of particle diffusiophoretic mobility Γp on the particle radius a and the Debye layer thickness κ−1. Solid line: ζp=−70 mV, Dotted line: ζp=−80 mV, Dashed line: ζp=−60 mV.
Fig. S4.
Calculated colloid transport in the presence of a solute gradient. (A) Solute distribution versus time. Particle distribution versus time for (B) small particles (diameter 0.06 μm) and (C) large particles (diameter 1.01 μm). Solid lines indicate calculations with fluid advection induced by solute gradient, whereas dashed lines indicate calculations without fluid advection.
Fig. S5.
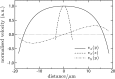
Representative flow field in capillary at x=15 μm from the open end and Dst=1,600 μm2 (i.e., 4Dst=80 μm). Shown is the longitudinal (vx) component of the flow field as a function of the distance from the center of the capillary in the transverse (y) and vertical (z) directions, and the transverse (vy) component in the transverse (y) direction.
Fig. S6.
Cross-section of dead-end channels filled with colloidal particles taken by confocal microscope (TCS SP5; Leica). Particle size: (A) 0.78 μm and (B) 1.01 μm; solution 0.02 mM NaCl. The particles were under stationary conditions. The final images are obtained by overlaying 300 consecutive images having time interval of 3 s. Line-averaged fluorescent intensity distribution, I(z)=∫I(y,z)dy along the vertical direction is also presented.
Fig. S7.
Numerical simulation results of the side view (x_–_z plane) of the particle migration along a dead-end channel driven by a solute gradient in the presence of flow advection; z=0 refers to the centerline of the channel, such that the images represent upper half of the channel. Particle size: (A) 0.06 and (B) 1.01 μm.
Fig. 3.
Interdiffusion of equimolar multicomponent solutes (ci = co = 2 mM) for inducing diffusiophoresis in a dead-end channel under constant osmolarity. (A) Fluorescent intensity distribution at 300 s and (B) trace of front position, xf=x(i=0.2Ii), for different solute configurations (blue: inner = KCl, outer = NaCl; red: inner = NaCl, outer = KCl). Results of a single solute with a gradient (NaCl: ci = 2 mM, co = 0.02 mM) are presented in black for comparison.
Fig. 4.
Control of colloidal particles in dead-end channels via a solute gradient for various applications. (A–D) Size-dependent particle sorting from mixture of particles in a dead-end channel driven by a solute gradient (NaCl: ci = 2 mM, co = 0.02 mM). The mixture consists of polystyrene particles having diameters of 0.21 and 1.01 μm dyed with different fluorophores. (A–C) Fluorescent images of (A) diameter = 0.21 μm particles and (B) 1.01 μm particles at t = 300 s, and (C) intensity distributions of A and B along the channel. D is an overlaid image of A and B. (E–G) Control of lipid vesicles for drug delivery applications. Fluorescent images of (E) SUVs (mean diameter ≈ 56 nm) and (F) LUVs (mean diameter ≈ 861 nm), and (G) intensity distributions of the vesicles along the channel at t = 300 s. (Scale bars: 50 μm.)
Similar articles
- Particle entrainment in dead-end pores by diffusiophoresis.
Battat S , Ault JT , Shin S , Khodaparast S , Stone HA . Battat S , et al. Soft Matter. 2019 May 15;15(19):3879-3885. doi: 10.1039/c9sm00427k. Soft Matter. 2019. PMID: 31021341 - Reversible Trapping of Colloids in Microgrooved Channels via Diffusiophoresis under Steady-State Solute Gradients.
Singh N, Vladisavljević GT, Nadal F, Cottin-Bizonne C, Pirat C, Bolognesi G. Singh N, et al. Phys Rev Lett. 2020 Dec 11;125(24):248002. doi: 10.1103/PhysRevLett.125.248002. Phys Rev Lett. 2020. PMID: 33412037 - Effect of polymer/surfactant complexation on diffusiophoresis of colloids in surfactant concentration gradients.
Yang A, McKenzie BE, Yi Y, Khair AS, Garoff S, Tilton RD. Yang A, et al. J Colloid Interface Sci. 2023 Jul 15;642:169-181. doi: 10.1016/j.jcis.2023.03.138. Epub 2023 Mar 24. J Colloid Interface Sci. 2023. PMID: 37003011 - Diffusiophoresis in Polymer and Nanoparticle Gradients.
Akdeniz B, Wood JA, Lammertink RGH. Akdeniz B, et al. J Phys Chem B. 2024 Jun 20;128(24):5874-5887. doi: 10.1021/acs.jpcb.4c00985. Epub 2024 Jun 5. J Phys Chem B. 2024. PMID: 38837230 Free PMC article. - Opto-thermal diffusiophoresis of soft biological matter: from physical principle to molecular manipulation.
Fukuyama T, Maeda YT. Fukuyama T, et al. Biophys Rev. 2020 Apr;12(2):309-315. doi: 10.1007/s12551-020-00692-7. Epub 2020 Apr 17. Biophys Rev. 2020. PMID: 32300983 Free PMC article. Review.
Cited by
- Steering particles via micro-actuation of chemical gradients using model predictive control.
McDonald MN, Peterson CK, Tree DR. McDonald MN, et al. Biomicrofluidics. 2023 Feb 1;17(1):014107. doi: 10.1063/5.0126690. eCollection 2023 Jan. Biomicrofluidics. 2023. PMID: 36742353 Free PMC article. - Unidirectional drying of a suspension of diffusiophoretic colloids under gravity.
Xu J, Wang Z, Chu HCW. Xu J, et al. RSC Adv. 2023 Mar 20;13(14):9247-9259. doi: 10.1039/d3ra00115f. eCollection 2023 Mar 20. RSC Adv. 2023. PMID: 36950706 Free PMC article. - Nanoelectrokinetic bufferchannel-less radial preconcentrator and online extractor by tunable ion depletion layer.
Lee S, Park S, Kim W, Moon S, Kim HY, Lee H, Kim SJ. Lee S, et al. Biomicrofluidics. 2019 May 30;13(3):034113. doi: 10.1063/1.5092789. eCollection 2019 May. Biomicrofluidics. 2019. PMID: 31186822 Free PMC article. - Diffusiophoresis promotes phase separation and transport of biomolecular condensates.
Doan VS, Alshareedah I, Singh A, Banerjee PR, Shin S. Doan VS, et al. bioRxiv [Preprint]. 2024 Feb 17:2023.07.03.547532. doi: 10.1101/2023.07.03.547532. bioRxiv. 2024. PMID: 37461689 Free PMC article. Updated. Preprint. - Diffusiophoresis-enhanced particle deposition for additive manufacturing.
Ghosh S, Lee S, Johnson MV, Hardin J, Doan VS, Shin S, Kalidindi SR, Lee J, Ault JT, Kong YL. Ghosh S, et al. MRS Commun. 2023 Dec;13(6):1053-1062. doi: 10.1557/s43579-023-00432-4. Epub 2023 Sep 14. MRS Commun. 2023. PMID: 38818251 Free PMC article.
References
- Howse JR, et al. Self-motile colloidal particles: From directed propulsion to random walk. Phys Rev Lett. 2007;99(4):048102-1–4. - PubMed
- Sabass B, Seifert U. Dynamics and efficiency of a self-propelled, diffusiophoretic swimmer. J Chem Phys. 2012;136(6):064508-1–15. - PubMed
- Palacci J, Sacanna S, Steinberg AP, Pine DJ, Chaikin PM. Living crystals of light-activated colloidal surfers. Science. 2013;339(6122):936–940. - PubMed
- Reinmüller A, Schöpe HJ, Palberg T. Self-organized cooperative swimming at low Reynolds numbers. Langmuir. 2013;29(6):1738–1742. - PubMed
- Anderson JL. Colloid transport by interfacial forces. Annu Rev Fluid Mech. 1989;21:61–99.
LinkOut - more resources
Full Text Sources
Other Literature Sources